Chemotherapy, which is considered one of the fundamental treatments for cancer, unfortunately, does not yield effective results when used as a standalone treatment. Hence, in this blog, I aim to examine the potential of fucoidan in boosting the efficacy of chemotherapy and its potential in mitigating the growth of cancer cells. I would like to introduce a study that investigated “Low-Molecular-Weight Fucoidan as Complementary Therapy of Fluoropyrimidine-Based Chemotherapy in Colorectal Cancer” by Ching-Wen Huanget et al.
In this study, the researchers aim to investigate the impact of low molecular weight fucoidan (LMWF) on improving the effectiveness of fluoropyrimidine-based chemotherapy in treating cancer. Additionally, the study aims to uncover the possible mechanisms by which LMWF enhances the anticancer efficacy of fluoropyrimidine-based chemotherapy.
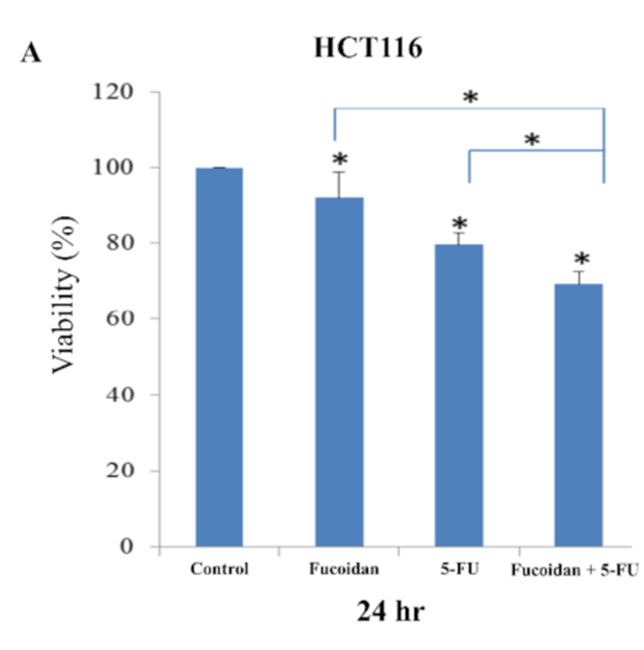
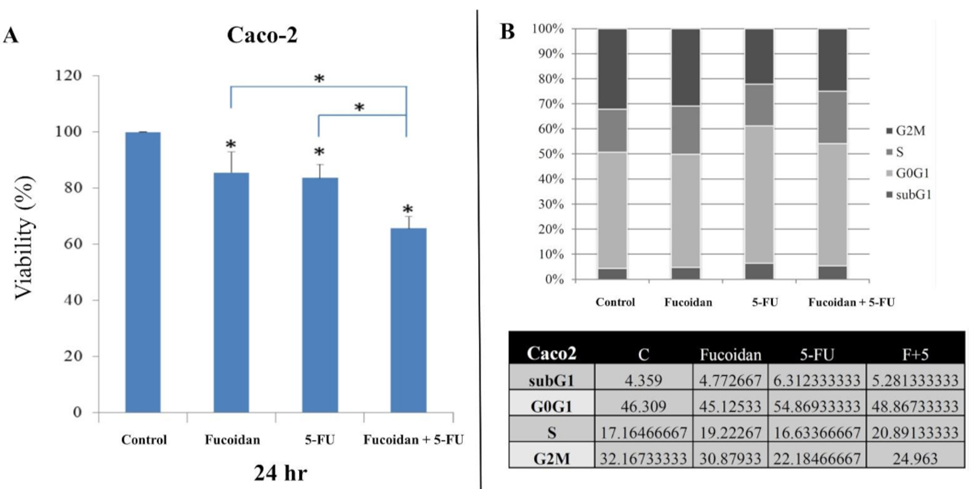
The initial step involved treating HCT116 and Caco-2 cells with LMWF and 5-FU. Following this treatment, various analyses were conducted to evaluate cell viability, cell cycle, apoptosis, and migration in both types of cells. The viability of HCT116 cells and Caco-2 cells was observed to be significantly lower 24 hours after treatment with LMWF or 5-FU, as depicted in Figures 1A and 2A. The combination of LMWF and 5-FU resulted in significantly lower cell viability in both HCT-116 cells and Caco-2 cells compared to control cells at the same 24-hour time point, as demonstrated in Figure 1A and Figure 2A. LMWF significantly enhanced late apoptosis induced by 5-FU. In Caco-2 cells, LMWF and 5-FU induced significantly slower apoptosis. The combination of LMWF-–5FU treatment resulted in a significant reduction in cell viability for both HCT116 cells and Caco-2 cells.
The use of 5-FU led to a significant increase in the sub-G1 and G0/G1 cell populations, along with a significant decrease in the proportion of G2M cells. However, the percentage of S-phase cells did not change significantly after treatment with 5-FU. The administration of LMWF and 5-FU in combination resulted in a significant elevation in the percentage of S-phase cells, in contrast to cells treated with either LMWF or 5-FU alone. In contrast, the cell cycle distribution in Caco-2 cells did not exhibit any noteworthy variations following the application of LMWF, 5-FU, or a combination of LMWF and 5-FU, as evidenced in Figure 2B.
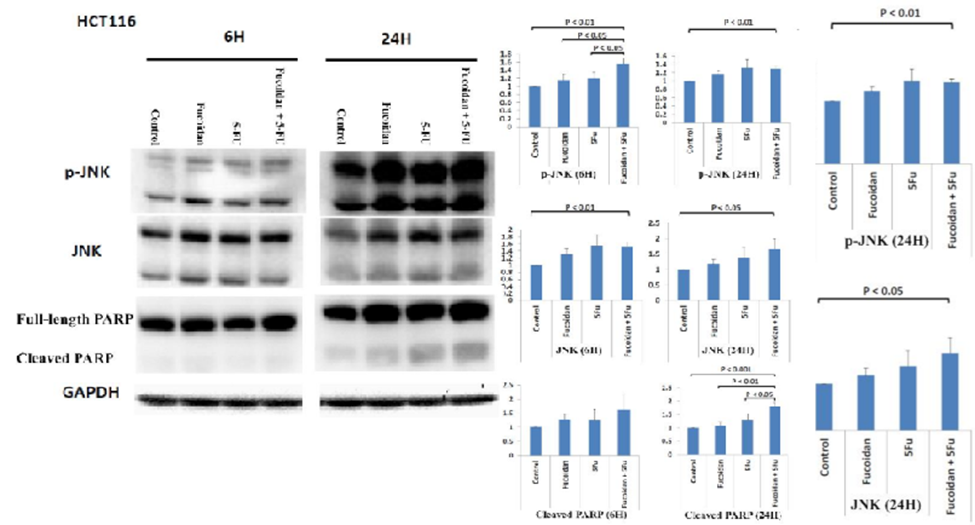
Next, they investigated whether the JNK signaling pathway was involved in apoptosis induced by the combined administration of LMWF and 5-FU. The combination treatment of LMWF and 5-FU led to a significant rise in the expression levels of phosphorylated JNK (p-JNK) protein in cells, particularly after 6 hours, when compared to cells treated individually with LMWF or 5-FU. (See Figure 3). However, total JNK protein expression was not significantly increased compared to cells treated with LMWF or 5-FU. The levels of cleaved PARP protein showed a significant increase in cells 24 hours after being treated with a combination of LMWF and 5-FU when compared to control cells and cells treated with only LMWF or 5-FU (as shown in Figure 3).
However, the combination of LMWF and 5-FU did not result in a significant increase in the expression of cleaved PARP protein after 6 hours of treatment. Therefore, based on these results, LMWF may enhance the effect of 5-FU on the apoptosis of HCT116 cells via the JNK signaling pathway. In HCT116 cells, LMWF influences the effects of 5-FU on cell survival through (1) induction of cell cycle arrest in the S phase and (2) late apoptosis mediated by the Jun-N-terminal kinase (JNK) signaling pathway.
The study investigated the potential involvement of the c-MET signaling pathway in the decrease of Caco-2 cell viability caused by the combined treatment of LMWF and 5-FU. The expression of the c-MET protein was significantly reduced in cells 6 hours after receiving a combination treatment of LMWF and 5-FU, in comparison to control cells and cells treated solely with LMWF or 5-FU. Furthermore, KRAS and p-ERK protein expression was significantly decreased in cells at 6 and 24 h after treatment with the combination of LMWF and 5-FU compared to control cells and cells treated with LMWF or 5-FU, respectively. Cells treated with the combination of LMWF and 5-FU displayed a noteworthy decrease in the expression of PI3K and p-AKT proteins at 6 and 24 hours, respectively. This decrease was significantly different from the expression levels observed in control cells and cells treated with either LMWF or 5-FU. As a result, the findings suggest that LMWF could potentially amplify the inhibitory effects of 5-FU on Caco-2 cell viability by targeting both the c-MET/KRAS/ERK and c-MET/PI3K/AKT signaling pathways.
Furthermore, the researchers looked into the potential involvement of the MMP-2 signaling pathway in the combined treatment of LMWF and 5-FU, and its impact on inhibiting the migration of HCT116 and Caco-2 cells. The levels of c-MET protein and MMP-2 protein were reduced in HCT116 cells and Caco-2 cells when treated with LMWF and 5-FU for 6 hours or 24 hours, as compared to the control cells. Therefore, according to these results, LMWF may enhance the effect of 5-FU on inhibiting migration in HCT116 cells and Caco-2 cells via the c-MET/MMP-2 signaling pathway.
To conclude, the study provided evidence that LMWF can enhance the anticancer properties of 5-FU by impacting the viability and migration of tumor cells in both HCT116 (KRAS mutant) and Caco-2 (KRAS wild type) colon cancer cell types.
Source: Int. J. Mol. Sci. 2021, 22(15), 8041; https://doi.org/10.3390/ijms22158041
