Chronic illnesses are the primary reason for global mortality, and the addition of polysaccharides to the diet is a successful and safe approach to treat chronic illnesses. Through this blog, an in-depth exploration will be conducted to examine the plausibility of a link between the increase in Lactobacillus and Allobaculum species and their impact on the regulation of intestinal flora. This unique perspective aims to offer new insights into the topic. So, I would like to introduce the beneficial effects of Laminaria japonica fucoidan (LJF) in the study “Fucoidan from Laminaria japonica Ameliorates Type 2 Diabetes Mellitus in Association with Modulation of Gut Microbiota and Metabolites in Streptozocin-Treated Mice”’ by Chenxi Zhang et al.
First, mice were divided into two groups: normal diet and high fat/carbohydrate diet. Three days after the third injection, mice in the HFD group with serum glucose >11.1 mM were defined as T2DM mice. T2DM mice were further divided into four groups: (i) model group (DM), gavaged with normal water; (ii) ME treatment group (ME), 200 mg/kg body weight of ME administered by oral gavage; (iii) High dose LJF group (HF), 500 mg/kg body weight of LJF is administered by oral gavage. (iv) low-dose LJF group (LF), 150 mg/kg body weight of LJF administered by oral gavage.
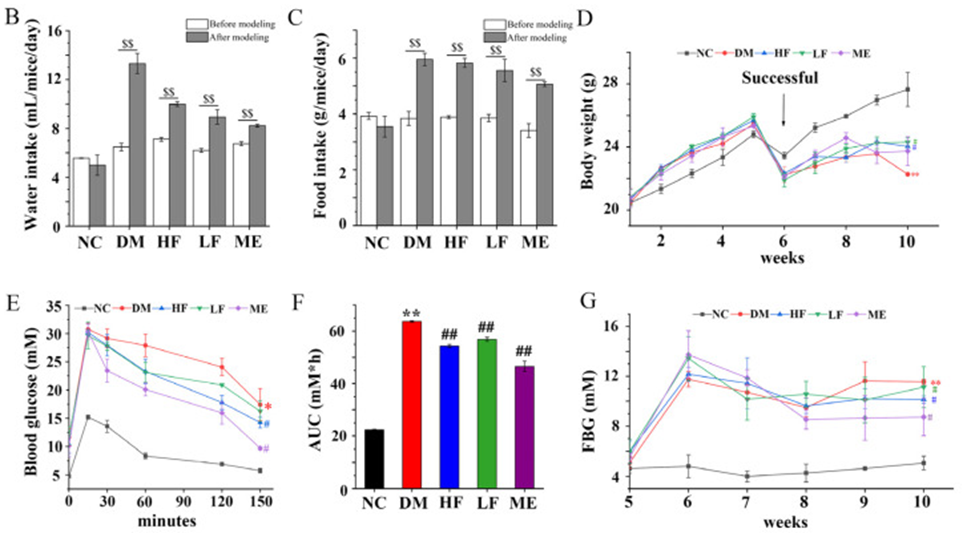
The administration of STZ (Streptozocin) resulted in increased food and water intake in the DM group, however, there was a noticeable decrease in body weight, as indicated in Figure 1B–D. The LF/HF group showed a decrease in water intake after 4 weeks of treatment, similar to the ME group, unlike the DM group. Regarding food intake, there was no difference between the LF/HF and DM groups, but food intake was decreased in the ME group. Body weight was higher in the LF, HF, and ME groups compared to the DM group, and no difference was found between them. The results of the OGTT indicated that individuals in the DM group had higher blood glucose levels compared to those in the NC group. These levels peaked at 15 minutes, as shown in Figure 1E.
In normal mice, blood glucose levels rapidly decreased to the initial state, whereas in the DM group, blood glucose levels decreased slowly. In contrast, blood glucose levels fell faster in mice fed LJF or ME, and the effect of LJF on blood glucose was dose-dependent. In comparison to the NC group, the DM group showed elevated AUC levels, while the T2DM mice that received LJF treatment demonstrated a greater reduction in AUC levels than those in the ME group, as seen in Figure 1F. FBG levels were higher in the DM group compared to the NC group, and LJF dose-dependently reduced his FBG levels in T2DM mice. (See Figure. 1G) Based on these results, LJF shows promise as a prebiotic agent for ameliorating the classic symptoms of T2DM.
The contents of serum TG, LDL-C, TC, ALT, AST, and FFA were increased in the DM group compared with the NC group, and GLP-1 and HDL-C levels were decreased. In contrast, low and high doses of LJF decreased serum LDL-C, TC, AST, TG, ALT, and FFA contents and increased HDL-C and GLP-1 levels in T2DM mice. This showed that LJF effectively ameliorated lipid metabolic abnormalities in his T2DM mice. Overall, high doses of LJF appear to have slightly better effects on lower doses.
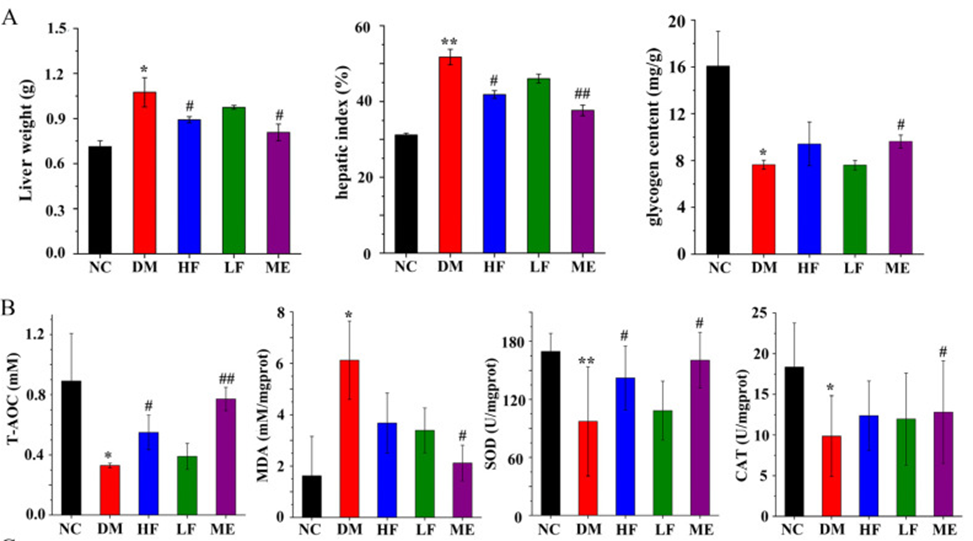
The DM group exhibited elevated liver weight and liver index when compared to the NC group, whereas liver glycogen levels were found to be decreased, as indicated in Figure 2A. LJF dose-dependently reduced liver weight and liver index in T2DM mice. As shown in Figure 2B, the contents of SOD, CAT, and T-AOC were decreased in the DM group compared with the NC group, and the MDA level was increased. In T2DM mice, high doses of LJF decreased MDA levels and increased T-AOC and SOD levels. The histological analysis revealed that the DM group had higher liver fat accumulation compared to the NC group. However, LJF (low-jitter frequency) effectively reduced fat accumulation in T2DM mice, particularly when administered at high doses.
The administration of LJF in both low and high doses resulted in reduced islet necrosis and β-cell damage, with the higher dose exhibiting a more pronounced protective effect on pancreatic islets. The results imply that LJF possesses the ability to preserve pancreatic islets, restore the equilibrium between insulin resistance and insulin sensitivity, and ameliorate abnormalities in insulin glucose metabolism in T2DM mice.
High doses of LJF in mice with T2DM led to an increase in the production of acetate, propionate, i-butyrate, i-valerate, and valerate. On the other hand, low doses of LJF resulted in an increase in the content of acetate, valerate, and valerate in the same mice. Meanwhile, ME caused an increase in acetate, butyrate, propionate, i-butyrate, i-valerate, and valerate in T2DM mice. High doses of LJF showed similar effects to ME on SCFAs suggesting their similarity to the regulation of gut microbiota.
Additionally, in mice with type 2 diabetes (T2DM), LJF reduced the levels of Proteobacteria but had no impact on Firmicutes and Bacteroidetes. On the other hand, ME decreased the levels of Proteobacteria and Firmicutes while increasing the levels of Bacteroidetes. This suggests that LJF and ME can modulate the composition of the gut microbiota.
Moreover, LJF was able to restore the dysbiosis in the gut microbiota of T2DM mice, leading to alterations in the profile of microbial metabolites. However, coccus exhibited a negative correlation. According to this study, LJF was found to be effective in improving various symptoms associated with T2DM, such as body weight, glucose and lipid metabolism, oxidative stress, and the integrity of pancreatic islets. It may also be related to the regulation of the gut microbiota. For instance, the population of Lactobacillus and Allobaculum species has been on the rise. Additionally, the remission of T2DM is closely linked to the involvement of microbial metabolites in the metabolic pathways of amino acids, glutathione, glyoxylic acid, and dicarboxylic acids. The study results suggested that LJF may be developed as a prebiotic drug for the prevention and treatment of T2DM.
Source: Foods. 2023 Nov; 12(22): 4132.doi: 10.3390/foods12224132
