In children and adolescents around the world, the most common primary malignant bone cancer is osteosarcoma (OS), and a combination of surgery and multiagent chemotherapy has led to a significant improvement in the 5-year cumulative survival rate of primary OS. Despite treatment, roughly 80% of OS patients see pulmonary metastases develop, and this is the leading cause of death. Hence, there is an urgent need for novel therapies that can combat the potential for metastasis in order to improve results for individuals diagnosed with OS.
Alternatively, it has been noted that sea cucumber fucoidan exhibits anticoagulant, antihyperglycemic, anti-inflammatory, and immunomodulatory properties. There is still a significant lack of knowledge regarding the fundamental ways in which sea cucumber Cucumaria frondosa fucoidan (Cf-Fuc) works to prevent the spread of cancer cells, which is unfortunate. This blog post will discuss the research paper by Minglei Zhang et al., titled “Sea cucumber Cucumaria frondosa fucoidan inhibits osteosarcoma adhesion and migration by regulating cytoskeleton remodeling.” This study examined how Cf-Fuc, affects the movement of human osteosarcoma epithelial cells (U2OS) in a lab setting, and the way it works.
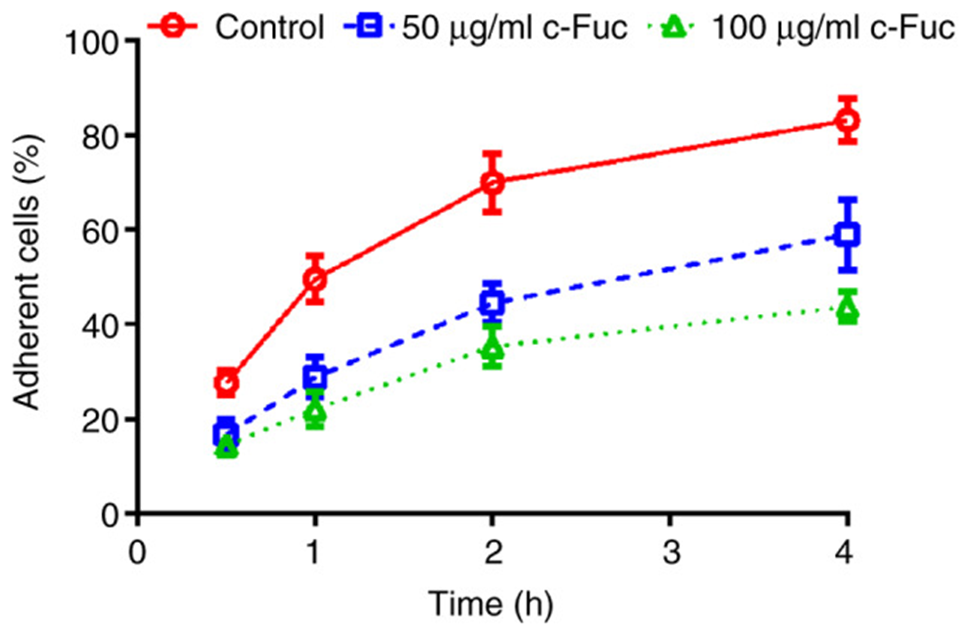
First, to evaluate the inhibitory effect of Cf-Fuc on U2OS cell adhesion and migration, they selected the non-cytotoxic concentrations of 50 and 100 µg/ml in the following experiments. As shown in Figure 1, the adhesion rate of untreated U2OS cells gradually increased and finally reached 83.2% at 4 h. Meanwhile, the adhesion rate of Cf-Fuc-treated cells increased slowly, indicating that Cf-Fuc treatment can inhibit U2OS cell adhesion when the Cf-Fuc concentrations were 50 µg/ml and 100 µg/ml.
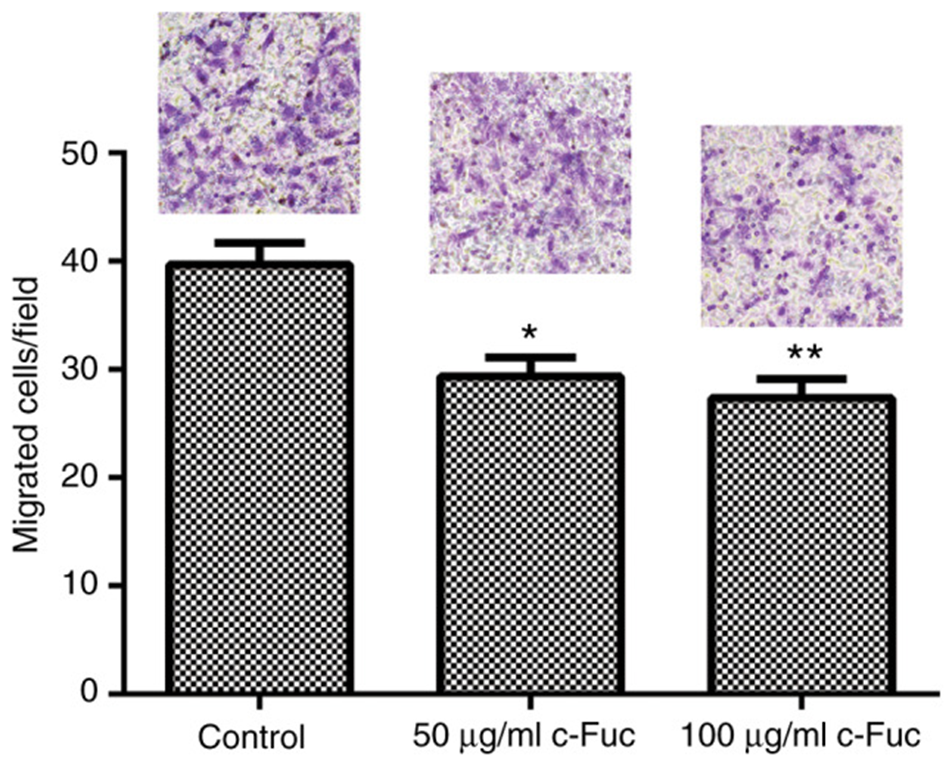
Next, the inhibitory effect of Cf-Fuc on the migration of U2OS cells was evaluated by transwell assay. Cf-Fuc strongly inhibited the migration of U2OS cells, and the number of migrating cells was significantly reduced compared to the control group (see Fig. 2). Next, the migration ability of Cf-Fuc-treated U2OS cells was quantitatively analyzed using NIH ImageJ. The cells in the control group showed strong migration ability, and the migration speed reached 64.8 µm/h. It is important to note that Cf-Fuc diminished the migration capacity of U2OS cells when compared to the cells in the control group. The migration distance of Cf-Fuc-treated cells was significantly reduced, and the migration speed was reduced to 28.5 µm/h and 18.1 µm/h at concentrations of 50 µg/ml and 100 µg/ml, respectively.
Cytoskeleton remodeling is a key event involved in cancer metastasis. In the process of cancer cell adhesion and migration, the polymerization of G-actin into F-actin filaments, which create cytoskeletal stress fibers, also contributes to the formation of lamellipodia, which play a crucial role in osteosarcoma metastasis. They further investigated the effect of Cf-Fuc on actin polymerization. Actin polymerization in U2OS cells increased in a time-dependent manner. However, the F-actin content of Cf-Fuc-treated cells was decreased compared with that of control cells.
The subsequent investigation delved into the influence of Cf-Fuc on cell adhesion signaling in U2OS cells. In comparison to the control group, treatment with Cf-Fuc notably reduced the phosphorylation of FAK and paxillin, indicating that Cf-Fuc could potentially hinder the migration of U2OS cells by modulating the FAK/paxillin cell adhesion signaling pathway.
Rac1, a small GTPase, plays an important role in remodeling the actin cytoskeleton. Therefore, they examined whether Cf-Fuc could inhibit the activation of Rac1 in U2OS cells. Compared with the control group, the levels of GTP-Rac1 (the activated form of Rac1) were reduced by 72% and 91% when treated with Cf-Fuc at concentrations of 50µg/ml and 100µg/ml, respectively.
Research indicates that the Rac1/PAK1/LIMK1/cofilin signaling pathway regulates how the actin cytoskeleton forms and contributes to cancer metastasis. Then, to clarify whether Cf-Fuc inhibits adhesion and migration of U2OS cells via the PAK1/LIMK1/cofilin signaling axis, we further examined the phosphorylation levels of PAK1, LIMK1, and cofilin. Treatment with Cf-Fuc at concentrations of 50 µg/ml and 100 µg/ml significantly inhibited phosphorylated PAK1, LIMK1, and cofilin compared with the control group. This finding implies a possible molecular process where Cf-Fuc prevents U2OS cells from sticking and moving by stopping the Rac1/PAK1/LIMK1/cofilin signaling pathway.
Overall, Cf-Fuc substantially decreased the OS cells’ ability to stick and move, diminished F-actin development, and reduced adhesion signaling by blocking the phosphorylation of FAK and paxillin. In addition, Cf-Fuc suppressed the PAK1/LIMK1/cofilin signaling pathway, leading to Rac1 activation and rearrangement of the actin cytoskeleton, suggesting a potential anti-metastatic action of Cf-Fuc.
The data demonstrated that Cf-Fuc substantially suppressed the adhesion and migration of OS cells, diminished F-actin formation, and also downregulated adhesion signaling by inhibiting the phosphorylation of FAK as well as paxillin. Cf-Fuc also inhibited the PAK1/LIMK1/cofilin signaling axis, which induces Rac1 activation and actin cytoskeleton reorganization, suggesting a possible anti-metastatic mechanism of Cf-Fuc.
Source: Oncol Rep. 2020 May 19;44(2):469–476. doi: 10.3892/or.2020.7614
