Oxidative stress has been suggested to be the main cause of Diabetic cardiomyopathy (DCM) development. This blog informs you of the study,” Low Molecular Weight Fucoidan Alleviates Cardiac Dysfunction in Diabetic Goto-Kakizaki Rats by Reducing Oxidative Stress and Cardiomyocyte Apoptosis” by Xinfeng Yu et al, that evaluates the protective effect of low molecular weight fucoidan (LMWF) extracted from Laminaria japonica on cardiac dysfunction in diabetic rats.
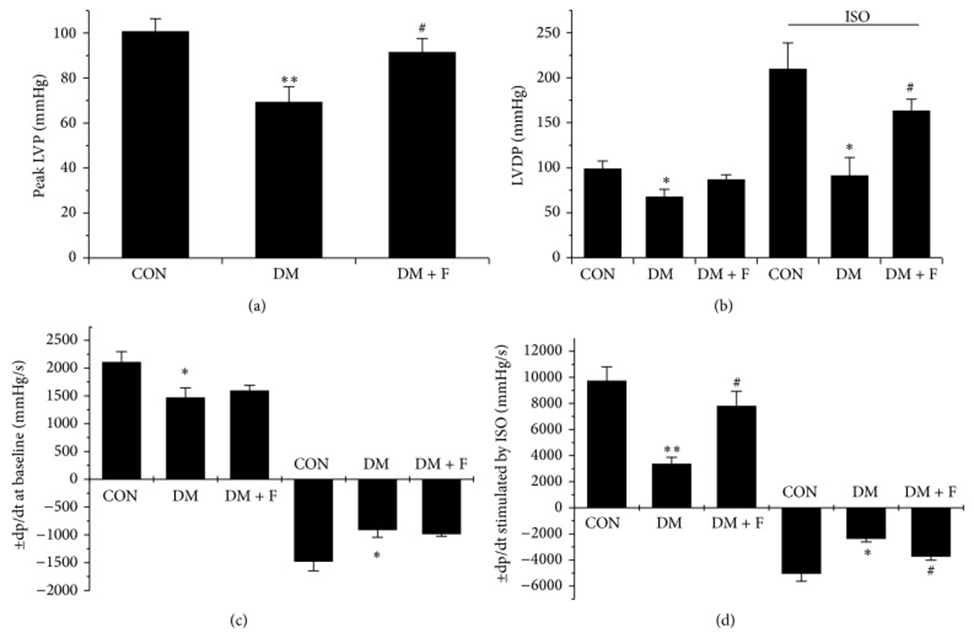
First, type 2 diabetic Goto-Kakizaki rats were left untreated or treated with LMWF (50 and 100 mg/kg/day) for 3 months. The establishment of the DCM model and the effect of LMWF on cardiac function were evaluated by echocardiography and isolated heart perfusion. As shown in Fig. 1, LMWF improved cardiac dysfunction by increasing myocardial contractility and compliance assessed by echocardiography and isolated heart perfusion.
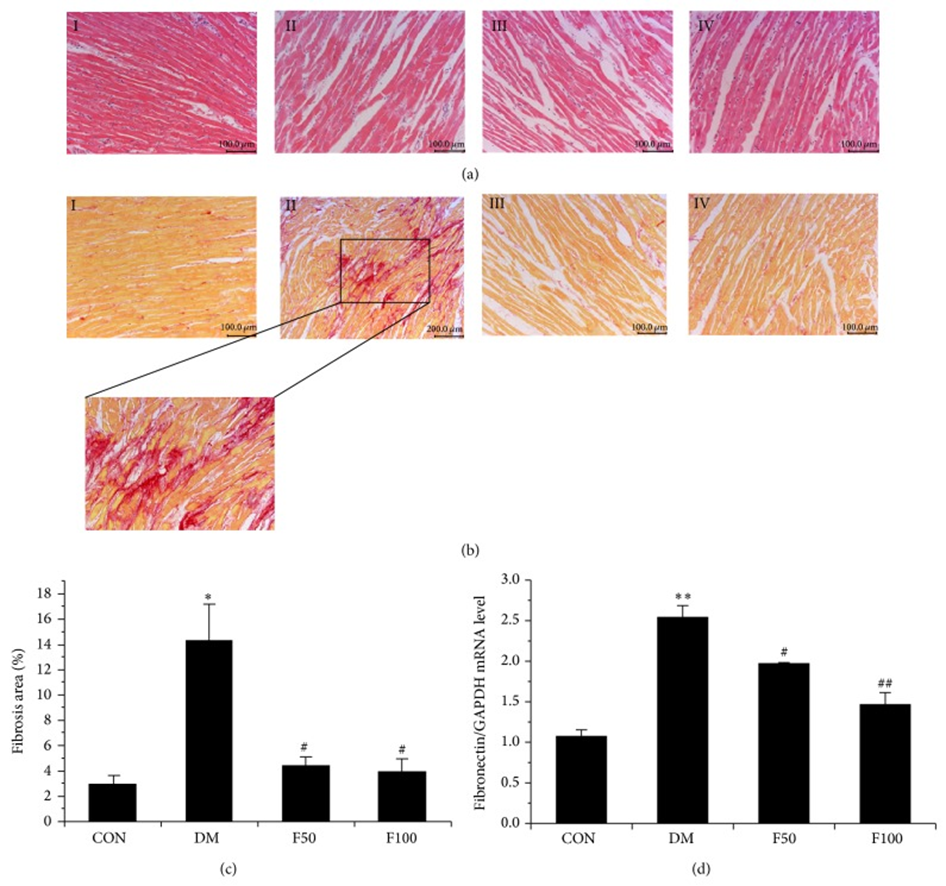
They investigated whether long-term administration of LMWF has a beneficial effect on myocardial structural changes, and the results revealed that, compared with the control group, obvious fibrosis and enlargement of the interstitial space were observed in the hearts of diabetic rats (Fig. 2(a) and 2(b)). Furthermore, compared with normal hearts, there was obvious accumulation of interstitial and perivascular fibrosis in the diabetic myocardium, but this was not notably observed in diabetic rats administered LMWF (Fig. 2(b) and 2(c)). Cardiac fibrosis in diabetic myocardium was further confirmed by measuring the expression of fibronectin, an extracellular matrix glycoprotein that plays an important role in myocardial dysfunction. As shown in Fig. 2(d), fibronectin mRNA levels were significantly increased in the hearts of GK rats compared with those of control rats (P < 0.05). Administration of LMWF significantly reduced the expression of fibronectin compared with the diabetic group, and in the magnified micrographs, the cardiomyocyte area in the left ventricular cross-section of diabetic rats was significantly increased compared with that of control rats (Fig. 3a). Further statistical analysis of cardiomyocyte hypertrophy in diabetic hearts showed that LMWF at concentrations of 50 and 100 mg/kg/day significantly reduced cardiomyocyte hypertrophy.
Cardiomyocyte death plays a crucial role in the development of DCM due to the loss of terminally differentiated cardiomyocytes. Inhibiting apoptosis with antioxidants leads to a significant reduction in diabetic cardiotoxicity. According to TUNEL assay, the brown-stained nuclei of apoptotic cardiomyocytes in the DM group were more intense and the proportion was greater than that in the control group. LMWF (50 or 100 mg/kg/day) administration significantly attenuated DNA fragmentation in diabetic cardiomyocytes. Furthermore, a Western blot was performed to detect the expression of apoptotic proteins. Bax is a mitochondrial protein that promotes cell apoptosis. PARP, a nuclear poly(ADP-ribose) polymerase, is involved in DNA repair in response to environmental stress, and cleavage of PARP serves as a marker for cells undergoing apoptosis. The expression of Bax and cleaved PARP was significantly enhanced in the DM group compared with the control group. Similarly, LMWF administration inhibited the increase in Bax and PARP cleavage in cardiac tissues of diabetic rats.
To investigate the cardioprotective mechanism of LMWF, lipid peroxide levels and antioxidant enzyme (SOD and CAT) activities were measured. Diabetic rats had elevated MDA levels and increased lipid peroxide accumulation. Consistently, the enzyme activity of antioxidant SOD was significantly decreased compared with the control group. LMWF administration enhanced SOD activity and suppressed MDA content in diabetic rats. CAT also showed a similar trend in diabetic rats, but without statistical significance. In addition to the changes in antioxidant enzymes, it has been suggested that abnormal activation of PKCβ caused by increased diacylglycerol (DAG) production by hyperglycemia acts in a positive feedback manner as an important contributor to ROS production in diabetic cardiomyocytes. Therefore, to investigate whether LMWF affects the changes in PKCβ expression, the expression of PKC in cardiac tissues of each group was detected by Western blotting. As a result, both PKCβ1 and PKCβ2 isotypes were significantly overexpressed in diabetic hearts, and this diabetes-specific change may be alleviated by LMWF intervention in diabetic rats.
Finally, they investigated the inhibitory effects of LMWF on ROS production, PKCβ overexpression, and cell apoptosis in cultured rat cardiomyocytes H9c2 loaded with high glucose (25 mM D-glucose). As a result, 20 μg/mL of LMWF significantly suppressed high glucose-induced PKCβ overexpression and ROS production in H9c2 cells. Furthermore, high glucose-induced cell death was also suppressed by 20 μg/mL of LMWF, as determined by the MTT assay.
As a result, LMWF inhibits oxidative stress and associated cardiomyocyte apoptosis in diabetic hearts by enhancing the activities of antioxidant enzymes and inhibiting PKCβ-dependent ROS production. Considering its efficacy and safety, LMWF may be a potential therapeutic agent for the prevention and treatment of DCM.
Source: J Diabetes Res. 2014 Nov 30;2014:420929. doi: 10.1155/2014/420929
