There is a substantial correlation between a high-salt diet (HSD) and several medical conditions, such as hypertension, chronic kidney disease, and autoimmune disorders, which are all significantly impacted. However, the mechanisms underlying HSD-induced inflammation and exacerbation of these diseases have not been fully elucidated.
This blog will discuss the study, “L-fucose and fucoidan alleviate high-salt diet-promoted acute inflammation” by Wenhua Li et al.
To begin the study of HSD’s pro-inflammatory impact, a standard acute inflammatory peritonitis model was created utilizing Zymosan A; after a period of 8 weeks during which the mice were given either a chow diet (CD) or a high-sucrose diet (HSD), the mice received a Zymosan A supplement by way of intraperitoneal injection (i.p.) and subsequent analysis using flow cytometry was carried out to assess the effects of the HSD.
HSD significantly increased the total cell count, neutrophil count, and monocyte/macrophage count in the peritoneal cavity at both 6 and 12 hours after injection. Furthermore, the expression of inflammatory cytokines, such as TNF-α, MCP-1, and IL-6, was also elevated after HSD treatment. These results strongly suggest that HSD not only promotes inflammatory cell infiltration but also increases the production of inflammatory cytokines in response to peritonitis. Taken together, these findings strongly suggest that HSD exacerbates the inflammatory response in mice with zymosan A-induced peritonitis.
Having established HSD’s pro-inflammatory role in peritonitis triggered by zymosan A, the goal was to explore the specific process behind this effect. The Na+ concentration within the ascites supernatant was measured at several time points as a preliminary step. Biochemical analysis revealed no significant difference in Na+ concentration between the HSD and CD groups. This indicates that HSD-induced inflammation promotion is not mediated by intraperitoneal Na+ accumulation. According to these results, the scientists suggested that HSD might accelerate inflammation in an indirect manner, possibly through alterations in the gut microbiota.
For this hypothesis’s verification, both HSD and CD mice were given broad-spectrum antibiotics for a month, leading to a depletion of their gut microbiota. After antibiotic treatment, the study evaluated the effect of the antibiotic on the response to peritonitis. After antibiotic treatment, the levels of several inflammatory cytokines, such as TNF-α, were increased in both the HSD and CD groups compared with non-antibiotic treatment, but there was no significant change in the total cell count, neutrophils, monocyte/macrophage infiltration, or expression of the major inflammatory cytokines TNF-α, MCP-1, and IL-6 at 6 and 12 hours after zymosan A injection, strongly suggesting that HSD-induced promotion of inflammation may be caused by dysbiosis of the intestinal microbiota.
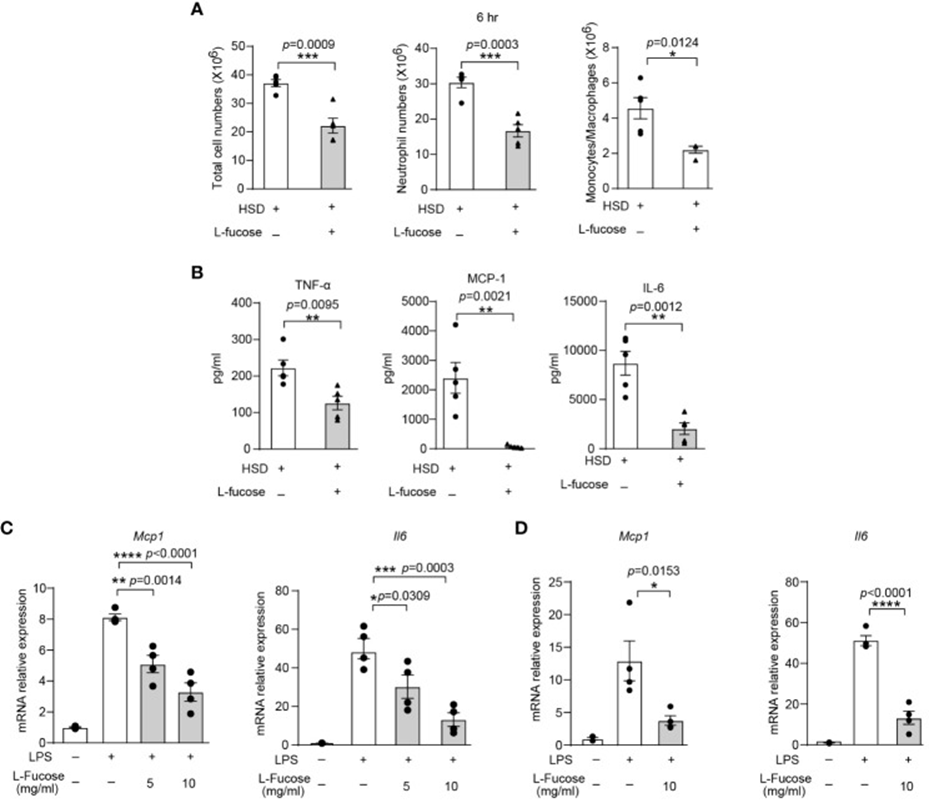
The anti-inflammatory impact of L-fucose was tested in living organisms via experiments using a zymosan A-induced peritonitis model in mice. Results: Mice administered HSD and L-fucose simultaneously showed significant decreases in the total number of cells, neutrophils, and monocytes/macrophages in the peritoneal cavity (See Figure 1A). Furthermore, the expression levels of inflammatory markers such as TNF-α, MCP-1, and IL-6 in the peritoneal fluid were also reduced (See Figure 1B). The researchers further evaluated the effects of L-fucose on RAW264.7 cells and peritoneal macrophages (PM). According to quantitative PCR results, the expression of Mcp1 and Il6 in RAW264.7 cells decreased when treated with L-fucose in a way that depended on the concentration (see Figure 1C). Similarly, the relative expression of Mcp1 and Il6 in PM was also reduced (See Figure 1D). The findings suggest that L-fucose can decrease inflammation in living organisms and in lab settings.
The above data demonstrate that L-fucose can reduce HSD-induced inflammation. Flow cytometry was utilized to examine the cells and cytokines in the peritoneal fluid of the fucoidan-treated group (FUC) and the HSD group, six hours after zymosan A injection, to evaluate fucoidan’s potential to alleviate inflammation caused by HSD. The results revealed that the total cell and neutrophil counts in the peritoneal fluid were lower in the fucoidan-treated group compared with the HSD group. The concentrations of the inflammatory cytokines TNF-α, MCP-1, and IL-6 within the supernatant of the FUC group were observed to be lower in comparison to those of the HSD group. Further in vitro experiments using RAW264.7 cells and PM were conducted, and the results further supported these findings. In conclusion, these findings imply that fucoidan, sharing anti-inflammatory characteristics with its primary element, L-fucose, can reduce inflammation intensified by HSD.
This study revealed that mice with lower levels of intestinal L-fucose experienced more intense inflammation when HSD was present. According to the data, L-fucose and fucoidan have the potential to suppress the inflammatory effects triggered by HSD. Moreover, finding potential drug candidates like L-fucose and fucoidan creates new possibilities for medical therapies.
Source: Front Immunol. 2024 Mar 26;15:1333848. doi: 10.3389/fimmu.2024.1333848
