This blog post aims to share the findings of the study titled “Investigation of Different Molecular Weight Fucoidan Fractions Derived from New Zealand Undaria pinnatifida in Combination with GroA Therapy in Prostate Cancer Cell Lines” by Xu Yang et al. In an effort to understand its antiproliferative effects on prostate cancer cells, the research utilized low-molecular-weight fucoidan (LMWF) extracted from Undaria pinnatifida (Wakame) and Sigma Fucoidan (FS). These compounds were combined with GroA, identified as a quadruplex-forming oligonucleotide aptamer (AS1411) and a powerful inhibitor of cell surface nucleolin.
Fucoidan is scientifically recognized for a range of physiological effects, including its antioxidant, anticoagulant, antiviral, and anticancer capabilities. Furthermore, the nucleolin inhibitor GroA (AS1411) represents a novel anticancer drug that has reached Phase II clinical trials. The molecule and related analogs inhibit proliferation and induce cell death in many cancer cell types, while having minimal effect on normal cells.
Initially, varying concentrations of LMWF (100, 200, and 300 µg/mL) and FS (500, 750, and 1000 µg/mL) were formulated for the treatment of PC-3 and DU-145 cells. Both LMWF and FS dose-dependently reduced cell proliferation and viability at 72 and 96 hours, whereas no effect was observed in control experiments. As shown in the PC-3 cell line, cell viability was 85-90% at 72 hours and approximately 82% at 96 hours. DU-145 cell viability showed a similar trend. Meanwhile, treatment with LMWF (300 µg/mL) significantly reduced cell viability at 72 and 96 hours in both PC-3 and DU-145 cell lines. The reduction in cell viability was also observed at 100 and 200 µg/mL LMWF. Next, we investigated how LMWF and FS, along with GroA (a strong nucleolin inhibitor), affected cancer cell growth and survival at reduced concentrations. The GroA/Cro level was consistently 10 µM in both cell lines, whereas LMWF was administered at 100, 220, and 300 µg/mL, and FS at 500, 750, and 1000 µg/mL.
As LMWF and GroA concentrations increased, cell proliferation consistently decreased in every group that was tested. This indicates that LMWF, irrespective of its low concentration, boosted GroA’s capacity to hinder cell proliferation in PC-3 and DU-145 cells as the dosage increased.
The combination therapy of FS and GroA yielded distinct results compared to LMWF when administered singularly. Viable cell counts following FS treatment at 500, 750, and 1000 µg/mL did not show significant inhibition (10%) compared to the control group. Cell viability after 72 hours of treatment with GroA was approximately 60%. However, no significant difference was observed when PC-3 cells were treated with GroA and FS compared to GroA alone. The DU-145 cell line showed similar results in all tested groups. In all groups, GroA alone reduced cell viability in both cell lines, even at higher FS concentrations. As a result, FS was unable to dose-dependently boost the inhibitory impact of GroA on cell growth and survival in PC-3 and DU-145 cells, despite being administered at very substantial concentrations.
The cell viability data revealed that LMWF and GroA together were significantly more effective at preventing PC-3 and DU-145 cell growth compared to using LMWF (at 100, 220, and 300 µg/mL) or GroA (at 10 µM) by themselves. However, only GroA-related effects were observed with the combined use of FS and GroA. It’s possible that FS was ineffective at 500, 750, and 1000 µg/mL, and therefore, higher concentrations of FS were required, which could account for this observation.
Subsequently, the examination focused on determining if the synergistic action of fucoidan and GroA resulted in elevated cell death rates in two types of prostate cancer cells. Cells were treated with either GroA or LMWF alone, or the two drugs in combination. The optimal incubation time for the combined treatment after each drug was 72 hours. Each medication demonstrated a notable suppression of cell proliferation at this stage. Triplicate measurements were taken for each experiment.
The DU-145 cells were subjected to treatment with LMWF, either independently or alongside GroA. After GroA treatment, the distribution of apoptotic cells showed a decrease in the percentage of viable cells of approximately 30%, while the percentages of early and late apoptotic cells increased by 23% and 5%, respectively. DU-145 cell groups treated with different concentrations of LMWF (100, 200, and 300 μg/mL) showed decreased cell viability compared to the control group. Additionally, in the group receiving combined treatment, higher LMWF concentrations correlated with a greater proportion of cells in both early and late apoptotic stages. Comparing GroA treatment alone with combined LMWF treatment, both early and late apoptotic cells increased, and the percentage of viable cells decreased with increasing LMWF concentration. Therefore, combined treatment with LMWF and GroA significantly altered cell viability and increased apoptosis in treated DU-145 cells.
Compared to the control (CroA), GroA resulted in a higher rate of apoptotic cell death in PC-3 cells. LMWF at increasing concentrations (100, 200, and 300 μg/mL) also increased apoptotic cell death. The total apoptotic rate increased with increasing LMWF concentration, and LMWF-induced cell apoptosis was increased by 20% compared with CroA alone. In addition, when cells were treated with both LMWF (300 μg/mL) and GroA, apoptotic cell death rose significantly compared to treatment with GroA alone, demonstrating a 35% increase in the apoptotic cell rate. Co-administration of 300 μg/mL LMWF and GroA was most effective in increasing apoptotic cell death. Thus, treatment with LMWF (100, 200, or 300 μg/mL) reduced cell death in PC-3 and DU-145 cells by approximately 20%. The co-administration of LMWF (at 100, 200, or 300 μg/mL) and GroA resulted in a significant elevation of apoptotic cell death across both cell lines, a greater effect than that observed with either agent alone. The data strongly imply that simultaneous administration of LMWF and GroA has the effect of suppressing cell viability and augmenting apoptotic cell death.
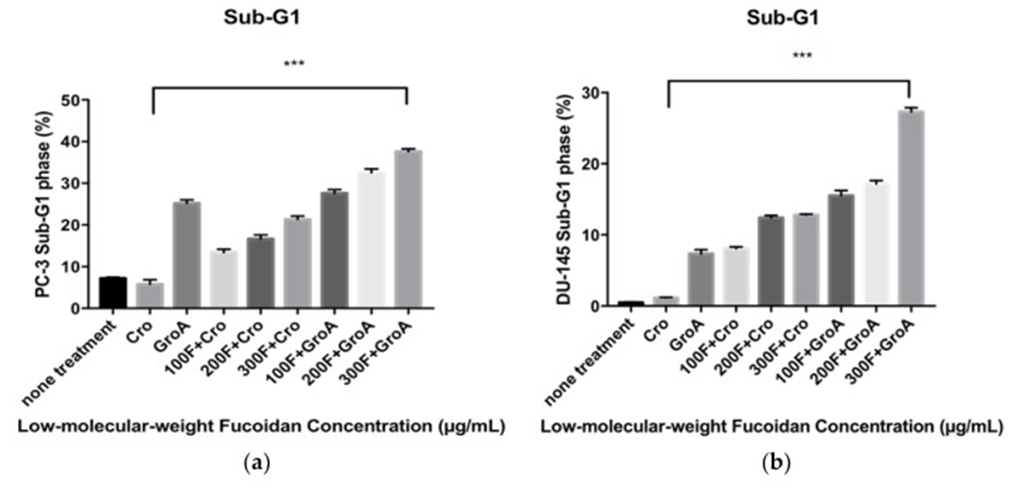
Flow cytometry analysis, employing PI DNA staining, was utilized to determine the changes in the cancer cells’ cell cycle subsequent to LMWF and GroA treatments. The proportions of the sub-G1 hypodiploid cells (which indicate the proportion of cell death) were estimated after 72 h of treatment for each cell line. By comparing the percentage (%) of sub-G1 fractions obtained after cells were treated with different concentrations (100, 200, and 300 µg/mL) of LMWF alone or in combination with Cro/GroA, the effectiveness of the drugs to increase cell death in different combination groups was estimated. This technique measures the index of fragmented DNA in apoptotic cells and also allows for the simultaneous assessment of cell-cycle parameters in surviving cells, such as their distribution within the cell cycle. The study also looked into whether cells were halted or prevented from progressing through any specific cell cycle stage.
The study first investigated how serum starvation impacted each cell line before evaluating the apoptosis-inducing effects of various Fucoidan preparations. The sub-G1 population was used as an index of apoptotic DNA fragmentation [32]. LMWF was found to increase the sub-G1 cell population in a dose-dependent manner, while it decreased the G0-G1 population in both PC-3 and DU-145 cell lines (see Figure 1). The combination of LMWF and GroA boosted apoptotic cell death in both PC-3 and DU-145 cell lines, evidenced by an increase in the sub-G1 population reaching 37.61% at the maximum LMWF concentration. The percentages of sub-G1 populations were higher in all the combined treatments compared to each of the treatments alone. The combination of LMWF and GroA, therefore, resulted in a synergistic effect. The cell cycle distributions, as measured, showed that both GroA and LMWF halted the sub-G1 phase, despite their distinct impacts on cellular metabolism. Furthermore, the accumulations of G0-G1 phase cells decreased dramatically to 37.31% in cells treated with a concentration of 300 µg/mL LMWF.
As a result, LMWF demonstrates a powerful effect on cell cycle progression within the two prostate cancer cell lines studied, causing an increase in the sub-G1 population and a decrease in the G0-G1 population. A synergistic effect is observed when LMWF is administered with GroA, leading to a more significant reduction in the G0-G1 population and a greater increase in the sub-G1 population.
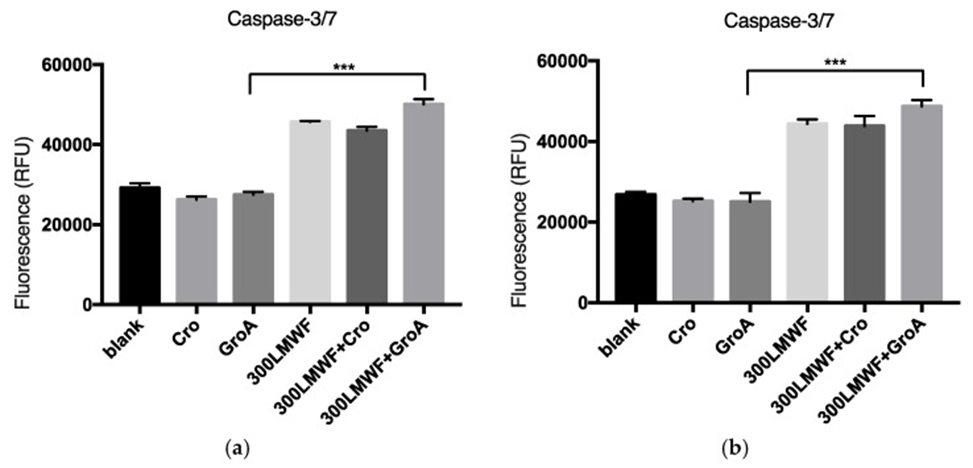
They explored if the cell death was caspase-dependent to learn more about it. For a duration of 72 hours, the researchers administered LMWF at a concentration of 300 µg/mL to cells, either independently or alongside GroA/CroA. Subsequently, they determined the levels of caspase 3/7 activity and analyzed how effectively it functioned within the different combination treatments. They found that LMWF increased the levels of caspase 3/7 activity in both PC-3 and DU-145 cell lines (see Figure 2). The combination of GroA and LMWF boosted the effect of LMWF, although GroA alone did not elevate caspase 3/7 levels. These results may suggest that LMWF controls the sequential activation of apoptotic cell death and that at least part of the cell death observed with LMWF and GroA is caspase-dependent.
This particular investigation’s findings indicated that, even when administered in reduced quantities, LMWF demonstrated a notable enhancement of GroA’s anti-proliferative capabilities. In addition, the combined effect of LMWF and GroA not only stops cell growth but also amplifies the cell death caused by each drug individually and by their combination. The cell death observed in this process is, at least in part, characterized as caspase-dependent apoptotic cell death. In summary, the combination of LMWF and GroA demonstrates a synergistic effect, boosting GroA’s anti-cancer activity and leading to a greater reduction in cancer cell growth and tumorigenicity across the tested cell lines. This suggests that LMWF could be a valuable addition to current prostate cancer treatments.
Source: Mar Drugs. 2018 Nov 18;16(11):454. doi: 10.3390/md16110454
