The spread of malignant tumors, known as metastasis, is a significant aspect of these tumors and is the primary reason why most cancer patients die. A crucial initial phase in the spread of cancerous tumors is metastasis through lymphatic vessels, which also serves as a predictor of patient outcome. Fucoidan, a naturally occurring sulfated polysaccharide, has been shown in recent studies to have anti-metastatic activity against various cancer types, and this is another point to consider.
According to the findings of multiple studies, both fucoidan and low-molecular-weight fucoidan have been found to display anti-angiogenic characteristics through the VEGF signaling pathway. However, the lymph-angiogenesis inhibitory effect of fucoidan has not yet been clarified. In this blog post, the study, “Fucoidan (U. pinnatifida sporophylls) inhibits lymph-angiogenesis by downregulating the expression of VEGFR3 and PROX1 in human lymphatic endothelial cells,” by Yazong Yang et al., is summarized.
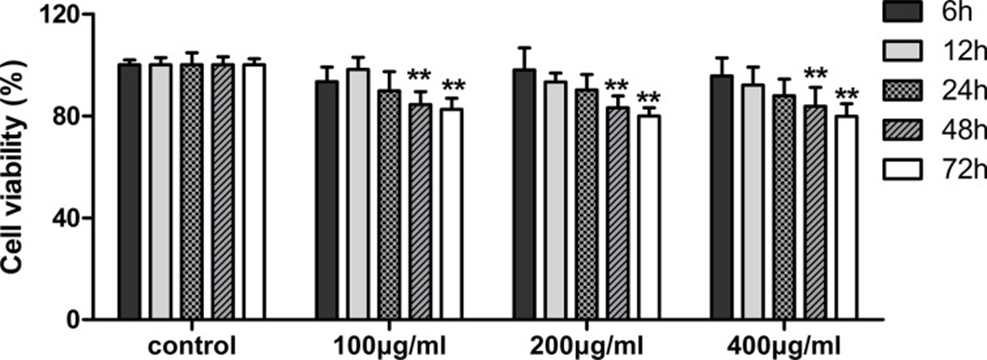
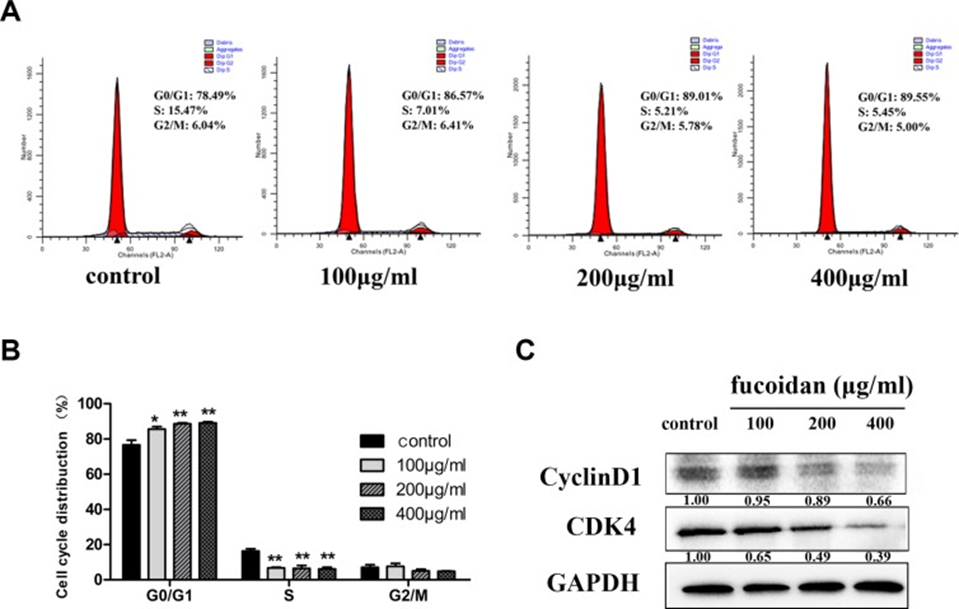
The MTT assay was utilized at the outset to determine the cell viability of human lymphatic endothelial cells (HLECs) exposed to fucoidan (0, 100, 200, and 400 μg/ml) across different time points. As shown in Figure 1, fucoidan significantly reduced cell proliferation after 48 and 72 hours of treatment. To clarify the mechanism of proliferation inhibition, HLECs were treated with fucoidan for 48 hours. Flow cytometry showed that fucoidan increased the number of cells in the G0/G1 phase and decreased the number of cells in the S phase (See Figures 2A and 2B). Furthermore, Western Blotting showed that fucoidan reduced the expression of CDK4 and cyclin D1, as shown in Figure 2C. Based on this information, fucoidan impedes cell proliferation and arrests the G1 phase of the cell cycle through the downregulation of CDK4 and cyclin.
Next, to investigate whether fucoidan inhibits HLEC migration in vitro, we used trans-well and wound scratch assays. In the trans-well assay, fucoidan-treated cells showed a significantly shorter migration distance compared to control cells (p < 0.01). In the wound scratch assay, the difference in cell migration distance after fucoidan treatment was visually demonstrated, with treated cells showing a shorter migration distance after 24 hours compared to control cells.
The study examined the in vitro tube formation of HLECs to determine if fucoidan possesses an anti-lymphangiogenic effect. After 24 hours of culture on Matrigel, HLECs formed entire tubes, but fucoidan-treated HLECs did not. Furthermore, the number of tube formations in the control group was significantly higher than in the fucoidan-treated group (p < 0.01). As a result, HLEC tube formation was effectively stopped by fucoidan treatment, with the degree of inhibition correlating to the dose. Both cell migration and tube formation are inseparably linked to the cytoskeleton. To examine whether fucoidan affects the distribution of microfilaments in well-differentiated epithelial cells (HLECs), cells were stained with green-fluorescent phalloidin. In fucoidan-treated HLECs, the distribution of microfilaments changed, resulting in altered cell polarity. Fucoidan can potentially control the restructuring of the cytoskeleton, which would then stop cells from moving.
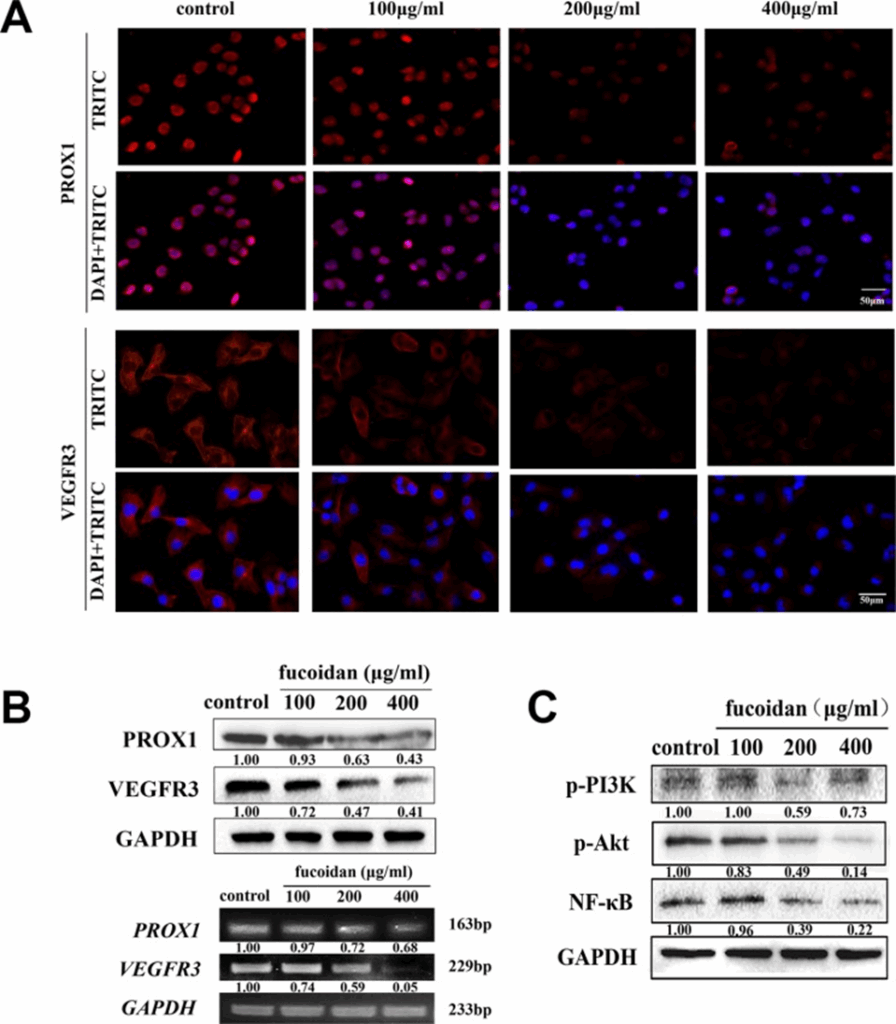
VEGFR3 and PROX1 are essential proteins that play key roles in lymph-angiogenesis and the movement of lymphatic endothelial cells (LECs). To investigate whether fucoidan acts as an inhibitor of metastasis and lymph-angiogenesis by reducing VEGFR3 and PROX1 expression, immunofluorescence and Western blotting were performed. In Figures 3A and 3B, the data suggested that higher fucoidan concentrations correlated with reduced levels of VEGFR3 and PROX1 mRNA and protein. Fucoidan at 200 and 400 μg/ml notably lowered VEGFR3 expression (p < 0.05), and it also significantly reduced PROX1 expression at the same concentrations.
Western blot analysis was performed to investigate whether fucoidan inhibited the activation of VEGFR3-induced signaling pathways. According to the results, fucoidan reduced the phosphorylation of PI3K and Akt, as well as the protein levels of NF-κB, in a manner that was dependent on the concentration, as shown in Fig. 3C.
We examined the capacity of fucoidan to generate lymphatic tubular structures within a co-culture system in order to further investigate its impact on inhibiting tumor lymph-angiogenesis. In the co-culture control group, more tubular structures were formed, and the lumens were tightly connected. The results showed that the HLEC co-culture system was significantly better at forming tubular structures compared to the monoculture (p < 0.05). In the co-culture system, fucoidan treatment effectively reduced the tubular structure formation of HLEC in a dose-dependent manner.
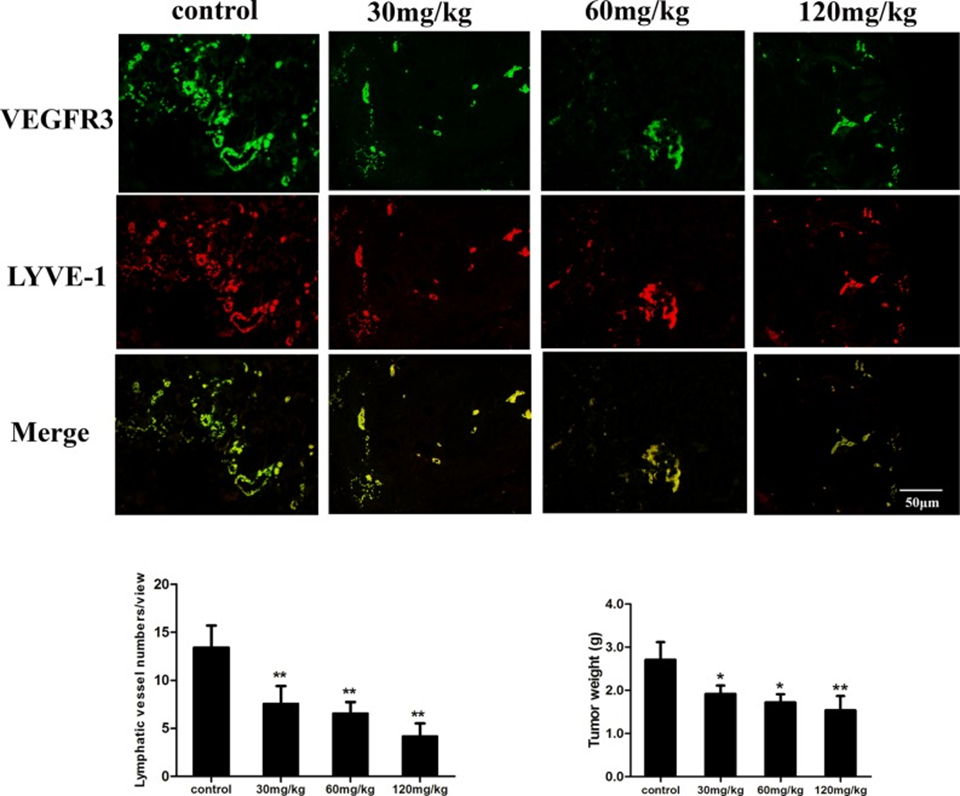
The antitumor and anti-lymph-angiogenic capabilities of fucoidan were assessed by inoculating mice with mouse hepatocellular carcinoma cells, specifically Hca-F cells, which are characterized by significant lymphatic metastasis activity. Tumor tissue was double immunofluorescent stained for the LEC markers VEGFR3 and LYVE-1. The results demonstrated that fucoidan (120 mg/kg) administration reduced the mean tumor microvascular diameter (LVD) from 13.4 ± 2.3 to 4.2 ± 1.3. As shown in Figure 4, the microvascular diameter (LVD) of mice treated with fucoidan was significantly reduced compared to the saline control group (p < 0.01). The mean tumor weight of fucoidan-treated mice was also significantly lower compared to the control group (p < 0.05 and p < 0.01). Based on the findings, it was evident that fucoidan has the capacity to impede tumor growth and lymph-angiogenesis within tumor tissue in a living environment.
The study findings suggest that fucoidan effectively inhibits lymph-angiogenesis in vitro, demonstrating antitumor and anti-lymph-angiogenic effects in vivo tumor models. Fucoidan suppressed cell proliferation through G1/G0 cell cycle arrest and exhibited antimigration and anti-tubule formation effects. The inhibitory effects of fucoidan on HLECs may be due to its ability to reduce PROX1 expression and suppress VEGFR3 activation, which may be associated with the inhibition of PI3K/Akt signaling in LECs. Therefore, fucoidan may be considered a potentially beneficial agent in the treatment of diseases and in novel approaches in clinical scenarios.
Source: Oncotarget. 2016 May 18;7(25):38025–38035. doi: 10.18632/oncotarget.9443
