Hypertension affects inflammatory pathological changes in the retina. In this blog, the study, “Fucoidan modulates SIRT1 and NLRP3 to alleviate hypertensive retinopathy: in vivo and in vitro insights” by Jing Li et al, that investigate whether sirtuin 1 (SIRT1) controls angiotensin II (Ang II)-induced hypertensive retinopathy and inflammation by regulating the activation of NOD-like receptor heat protein domain-related protein 3 (NLRP3) inflammasome, and the potential protective effects of fucoidan (FO) in mouse retinal endothelial cells (mRECs) and mouse retina.
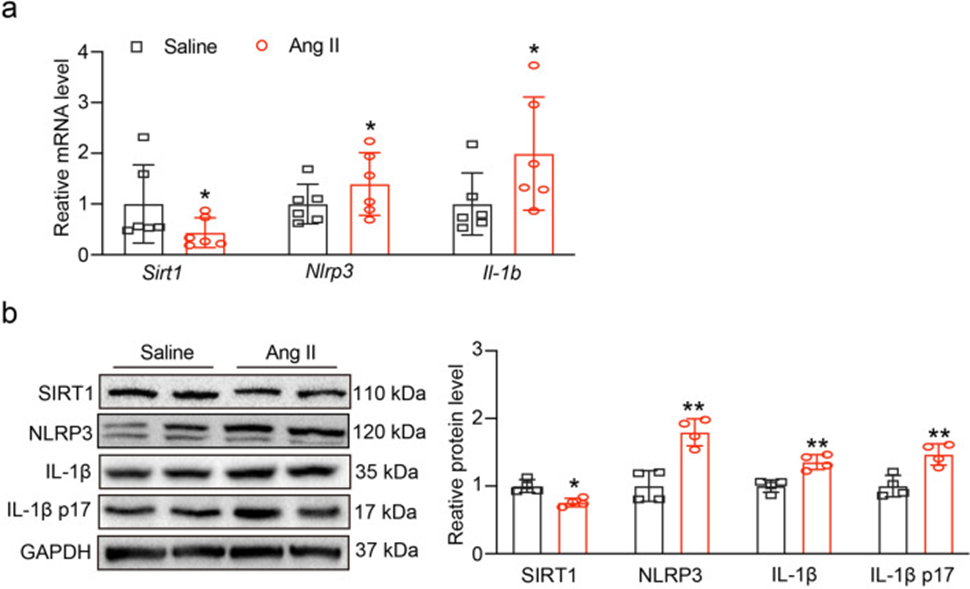
First, after 3 weeks of angiotensin II (3000 ng/kg/min) or saline administration, the mRNA levels of Sirt1, Nlrp3, and Il1b were evaluated. As shown in Fig. 1a, angiotensin II administration decreased the mRNA level of Sirt1 but significantly increased the mRNA levels of Nlrp3 and Il1b. Western blot results shown in Fig. 1b, c showed that the expression of SIRT1 was also decreased, and the expression of NLRP3 and IL-1β and its active form, IL-1β p17, was increased. These results suggested that SIRT1 and NLRP3 inflammasomes may be involved in angiotensin II-induced myocardial infarction.
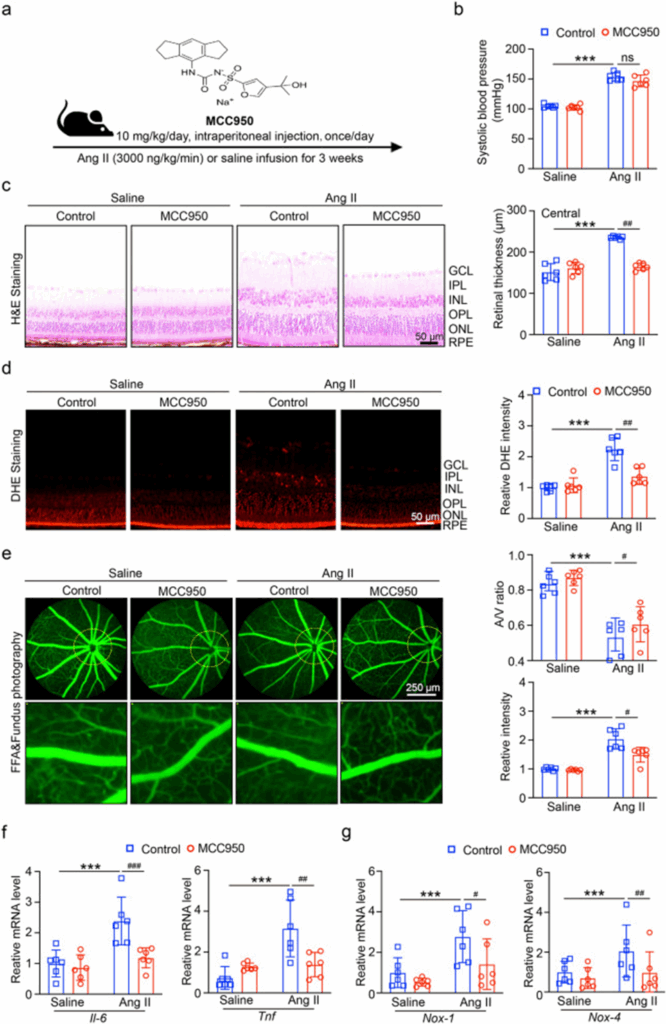
To investigate the role of NLRP3 inflammasome in angiotensin II-induced ventricular tachycardia (HR), mice were treated with the NLRP3 inhibitor MCC950 (10 mg/kg/day, i.p.) 1 day before angiotensin II injection (Fig. 2a). After MCC950 administration, systolic blood pressure (SBP) did not decrease in angiotensin II-injected mice (Fig. 2b). H&E staining showed that NLRP3 inhibition attenuated angiotensin II-induced central retinal thickening (Fig. 2c). We then detected oxidative stress in each group. As shown in Fig. 2d, DHE staining showed that NLRP3 inhibition significantly reduced angiotensin II-induced ROS production. Furthermore, angiotensin II (Ang II)-induced retinal arteriolar structural damage (indicated by angina, tortuosity, and exudation) was significantly improved in the retinas of MCC950-treated mice compared with the PBS-treated control group (Fig. 2e). The RT-qPCR results in Fig. 2f, g showed that NLRP3 inhibition reduced the mRNA expression levels of NADPH oxidases (Nox1 and Nox4) and inflammatory enzymes (Il6 and Tnf) in angiotensin II-treated mice.
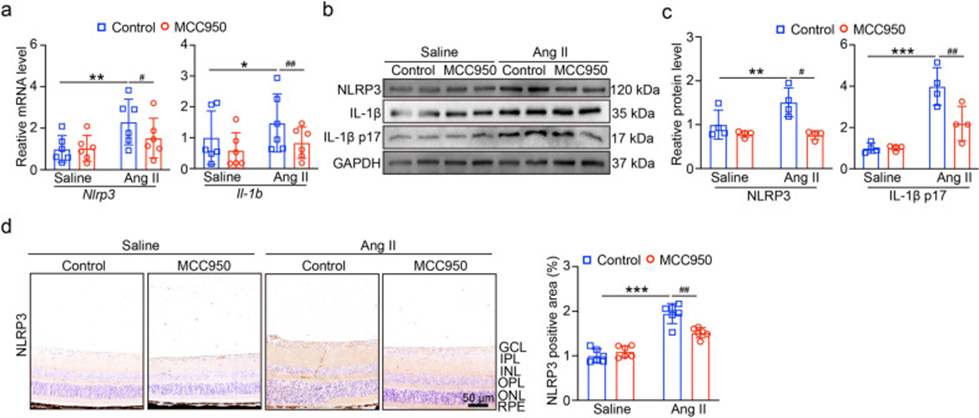
Furthermore, they investigated the levels of NLRP3 and IL-1β mRNA, and as shown in Fig. 3a, MCC950 administration suppressed the levels of Nlrp3 and IL-1b mRNA in Ang II-injected mice. Similarly, MCC950 significantly suppressed NLRP3 protein levels and IL-1β p17 secretion under Ang II infusion (Fig. 3b, c). Immunohistochemical staining of NLRP3 in Fig. 3d revealed that NLRP3 was most highly expressed in the ganglion cell layer (GCL), inner plexiform layer (IPL), and inner nuclear layer (INL) of the retina. After Ang II administration, the levels were elevated in the outer layer, whereas NLRP3 expression was significantly suppressed after MCC950 administration. The findings of this study indicate that the best option to block Ang II-induced HR and NLRP3 inflammasome activation is the NLRP3 protein inhibitor MCC950.
Furthermore, they investigated the levels of NLRP3 and IL-1β mRNA, and as shown in Fig. 3a, MCC950 administration suppressed the levels of Nlrp3 and IL-1b mRNA in Ang II-injected mice. Similarly, MCC950 significantly suppressed NLRP3 protein levels and IL-1β p17 secretion under Ang II infusion (Fig. 3b, c). Immunohistochemical staining of NLRP3 in Fig. 3d revealed that NLRP3 was most highly expressed in the ganglion cell layer (GCL), inner plexiform layer (IPL), and inner nuclear layer (INL) of the retina. After Ang II administration, the levels were elevated in the outer layer, whereas NLRP3 expression was significantly suppressed after MCC950 administration. The findings of this study indicate that the best option to block Ang II-induced HR and NLRP3 inflammasome activation is the NLRP3 protein inhibitor MCC950.
To evaluate whether SIRT1 plays a regulatory role in NLRP3 inflammasome activation, mRECs were treated with SRT1720 (0.5 μM) for an additional hour, followed by Ang II (100 nM) for 24 hours. After SRT1720 treatment, both the mRNA and protein levels of Nlrp3, and the mRNAs of IL1b and IL-1β p17 protein expression were decreased in Ang II-treated cells. DCFH-DA staining showed that ROS production was decreased in Ang II-treated cells after SRT1720 treatment. Thus, upregulation of SIRT1 may play a role in suppressing Ang II-induced NLRP3 inflammasome activation and ROS production.
To evaluate the therapeutic effect of fucoidan (FO), cells were treated with FO (60 μg/ml) for another 4 h, followed by Ang II (100 nM) for 24 h. The results showed that ROS production was reduced in Ang II-treated cells after FO treatment. Next, the level of Sirt1 mRNA was tested, and FO treatment increased Sirt1 mRNA levels. Similarly, SIRT1 protein expression was upregulated after FO treatment. Furthermore, Nlrp3 mRNA and protein levels were tested, and both Nlrp3 mRNA and protein expression levels were decreased in Ang II-treated cells. These results suggest that FO may upregulate SIRT1 expression and reduce NLRP3 activation.
Mice were administered FO (300 mg/kg/day) one day before Ang II injection. H&E staining showed that FO administration reduced Ang II-induced central retinal thickening. Next, DHE staining showed that FO administration significantly inhibited Ang II-induced ROS production. f RT-qPCR results showed that FO reduced the mRNA expression levels of Nox1, Nox4, Il6, and Tnf in Ang II-injected mice.
They next examined the levels of Sirt1, Nlrp3, and Il1b mRNA, and found that FO administration increased Sirt1 mRNA levels and suppressed Nlrp3 and Il1b mRNA levels in Ang II-infused mice. Similarly, FO significantly increased SIRT1 and suppressed NLRP3 protein levels and IL-1β p17 secretion under Ang II infusion.
The study demonstrated that angiotensin II (Ang II) infusion induces heart rate (HR) and dysfunction by decreasing SIRT1 and overexpressing NLRP3 inflammasome activation. We also found that FO therapy increases SIRT1 expression while decreasing NLRP3 activation, retinopathy, and dysfunction. These results suggest the possibility of an intensive treatment plan for HR dysfunction.
Source: J Transl Med. 2024 Feb 15;22:155. doi: 10.1186/s12967-024-04877-6
