The endoplasmic reticulum is an essential organelle for maintaining homeostasis, responsible for the post-translational modification and proper folding of membrane and secretory proteins. However, excessive or sustained endoplasmic reticulum stress is too much for the Unfolded Protein Response (UPR) to handle, and cells ultimately undergo apoptosis. It has been reported that apoptosis can lead to cell loss or tissue dysfunction, leading to the development of various diseases, and can also disrupt the balance of redox reactions and activate the release of reactive oxygen species (ROS).
Fucoidan, a sulfated polysaccharide extracted from brown algae, exhibits anticancer activity, but the effect and mechanism of fucoidan-induced apoptosis via endoplasmic reticulum (ER) stress remain unclear.
In this blog, I would like to inform you of the study, “Fucoidan induces Toll-like receptor 4-regulated reactive oxygen species and promotes endoplasmic reticulum stress-mediated apoptosis in lung cancer” by Hsien-Yeh Hsu et al, that fucoidan from Fucus vesiculosus will be shown to prevent tumor formation and reduce tumor size in Lewis lung carcinoma LLC1 xenografted male C57BL/6 mice.
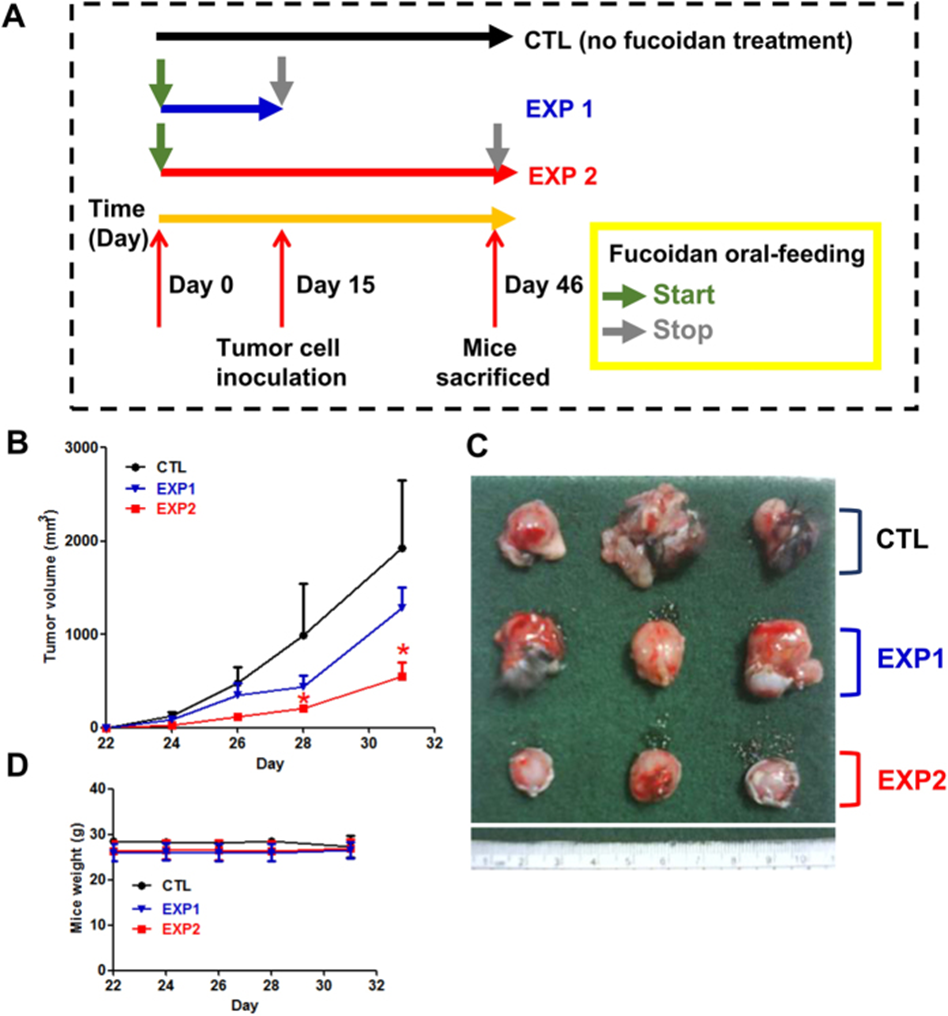
First, mice were divided into three groups: group 1 was ddH2O control (CTL), group 2 received only fucoidan before LLC1 inoculation and stopped oral fucoidan intake on day 15 (EXP 1), and group 3 received fucoidan throughout the entire experimental period (EXP 2, Figure 1A). The tumor volume of mice in EXP 2 was significantly reduced compared to mice in EXP 1 (Figure 1B and C). Thus, fucoidan treatment in EXP 1 was less effective at inhibiting tumor growth compared to continuous fucoidan treatment in EXP 2. In addition, small differences in mouse body weight were observed between mice that received fucoidan (EXP 1 and EXP 2) and ddH2O control (CTL, Figure 1D). Taken together, these results indicate that continuous oral intake of fucoidan exerts the greatest effect on preventing and suppressing tumor formation.
Next, the effects of fucoidan intake on LLC1 tumor-bearing mice were investigated in vivo. Tumor volume was significantly reduced in mice that consumed fucoidan. In contrast, a smaller difference was observed in the body weight of mice that consumed fucoidan and the ddH2O control group, indicating that this dose of fucoidan does not have a toxic effect on the C57BL/6 mouse model. We then harvested the tumor lesions of the mice and analyzed the expression of ER stress-related proteins. ATF4 and CHOP protein levels were increased in LLC1-bearing mice that consumed fucoidan compared to the control group, indicating that fucoidan-induced ER stress-related proteins in tumors in vivo.
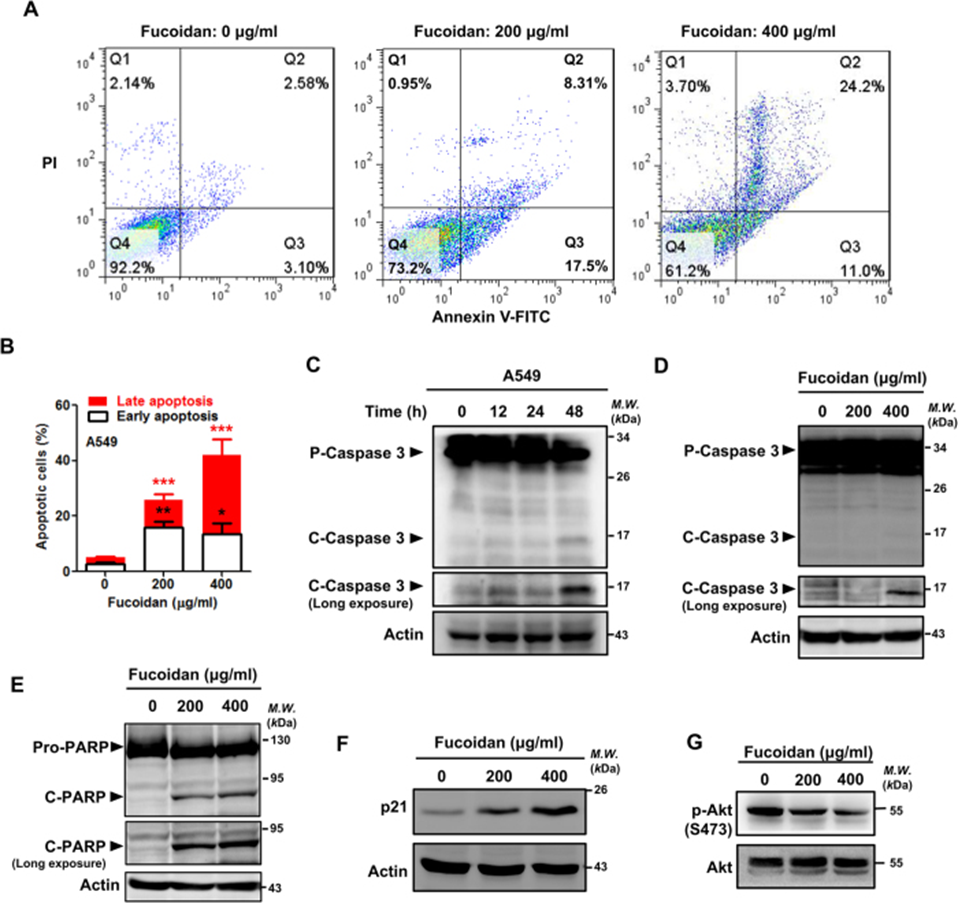
They investigated whether fucoidan induces apoptosis in A549 and CL1-5 lung cancer cells using FACS analysis with propidium iodide (PI)/annexin V-FITC double staining. As shown in Fig. 2A, B, after 48 h of fucoidan treatment, apoptotic cells increased from 5% to 40–60% and from 3% to 18–50% in A549 and CL1-5 cell lines, respectively. Furthermore, using western blot analysis of caspase activity, they demonstrated that fucoidan induces apoptosis in A549 and CL1-5 cells. This was indicated by the dose- and time-dependent expression of the proapoptotic proteins caspase 3 and poly ADP ribose polymerase (PARP) (Fig. 2C–E). Furthermore, using western blot assay and FACS analysis, they also found that fucoidan activated caspase 3 and PARP and induced apoptosis in LLC-1 cells. They also analyzed the effect of fucoidan on cell cycle distribution in lung cancer cells. Compared with control cells, fucoidan treatment (200 μg/ml, 48 h) increased the percentage of sub-G1 and G1 phases in A549 cells from 1% to 4% and from 63% to 85%, respectively. These results are comparable to those of another report that evaluated the biological activity of low-dose fucoidan. Furthermore, they confirmed that fucoidan upregulated the expression of cell cycle-related proteins, including p21, in both A549 and CL1-5 cells (Figure 2F). In contrast, fucoidan inhibited AKT phosphorylation in treated cells (Figure 2G).
After fucoidan treatment, the expression of GRP78 was increased in A549 and CL1-5 cells, suggesting that GRP78 is a gatekeeper of fucoidan-mediated ER stress activation. Fucoidan also induced phosphorylation of the ER membrane protein PERK in a time-dependent manner. They then systematically analyzed the molecules downstream of PERK. Fucoidan-induced phosphorylation of eIF2α and the expression of ATF4 and CHOP was increased in these cells in a time-dependent manner. The expression of CHOP was increased in CL1-5 cells treated with fucoidan for 24 h. These results indicate that (i) fucoidan induces ER stress via activation of the PERK/eIF2α/ATF4/CHOP axis, and (ii) activation of these axes is associated with apoptosis in some cancer cells and is involved in the inhibition of cell viability.
To further confirm the role of ATF4 in ER stress-induced cell death, we performed ATF4 knockdown experiments in A549 cells. Western blot analysis showed that ATF4 knockdown reduced ER stress-induced ATF4 expression. When they examined the activation of the ATF4/CHOP axis by ER stress, we found that ATF4 knockdown abolished fucoidan-induced ATF4-mediated CHOP expression. This suggests that ATF4 plays a key role upstream of ER stress-induced CHOP in the presence of fucoidan. To further explore the role of ATF4 in lung cancer cell survival after fucoidan treatment, we performed crystal violet staining assays to examine cell viability and proliferation. Fucoidan effectively inhibited cell viability and proliferation in a dose- and time-dependent manner. These results suggest that ATF4 may act as a fucoidan-mediated cell death molecule involved in ER stress in lung cancer.
Using the ROS scavenger N-acetyl-L-cysteine (NAC), they also found that ROS generation was involved in fucoidan-induced ER stress-mediated apoptosis. Furthermore, they demonstrated that fucoidan-induced ROS and CHOP expression were attenuated via Toll-like receptor 4 (TLR4) knockdown. The study is the first to identify a new mechanism for the antitumor activity of fucoidan. They showed that fucoidan inhibits tumor survival, induces apoptosis, and suppresses lung cancer cell progression by activating the TLR4/ROS/ER stress axis and the downstream PERK-ATF4-CHOP pathway. Together, these results indicate that fucoidan is a potential preventive and therapeutic agent for lung cancer that acts via activation of the ROS-dependent ER stress pathway.
Source: Sci Rep. 2017 Mar 23;7:44990. doi: 10.1038/srep44990

The study highlights the potential of fucoidan in reducing tumor volume through continuous intake. It is interesting to note that the treatment did not cause significant changes in body weight, suggesting minimal toxicity. The findings emphasize the importance of sustained fucoidan administration for optimal results. How does fucoidan specifically induce apoptosis via endoplasmic reticulum stress in tumor cells? WordAiApi
Fucoidan exerts its antitumor function by modulating the ER stress cascade, and exerts extensive control over ER stress by attenuating the cell survival cascade and activating the cell apoptosis cascade in cancer cells.