Fucoidan, a sulfated polysaccharide extracted from brown algae (Fucus vesiculosus), demonstrates diverse immunomodulatory effects, such as antiviral and antitumor activities. However, the effects of fucoidan in vivo, particularly its adjuvant effect on antitumor immune responses, have not been fully investigated. This blog post discusses a study by Jun-O Jin and colleagues, titled “Fucoidan Can Function as an Adjuvant In Vivo to Enhance Dendritic Cell Maturation and Function and Promote Antigen-Specific T Cell Immune Responses“. This study investigates the effect of fucoidan on the activity of splenic dendritic cells and its characteristics as an adjuvant in a biological system.
First, they evaluated whether fucoidan can induce the maturation of mouse dendritic cells in vivo. Fucoidan (10 mg/kg) was administered intraperitoneally to C57BL/6 mice, and the observation period lasted 24 hours. Fucoidan treatment significantly increased the expression of CD40, CD80, CD86, and MHC class II in splenic CD11c+ cDCs. Subsequently, we assessed the impact of fucoidan on CD8α+ and CD8α− cDC subpopulations one day after it was introduced. Fucoidan treatment significantly increased the expression of CD40, CD80, CD86, and MHC class II in both CD8α+ and CD8α− cDCs. The data indicate that fucoidan treatment promotes the maturation of splenic cDCs in living organisms.
To investigate whether fucoidan affects cytokine production, serum and spleens were collected from C57BL/6 mice 3 hours after fucoidan administration, and inflammatory cytokines were measured. Fucoidan administration induced increases in IL-6, IL-12p40, and TNF-α mRNA levels in splenocytes, but not in IL-23p19 mRNA levels. Serum IL-6, IL-12p70, and TNF-α levels also dramatically increased in fucoidan-treated mice. For a focused analysis of cytokines from cDCs, Lenease-CD11c+ cDCs were obtained from splenocytes two hours after fucoidan was given, using a cell sorter, and subsequently cultivated in culture medium for an additional four hours.
Fucoidan treatment led to a substantial rise in IL-6, IL-12p70, and TNF-α production within the culture medium. Furthermore, CD11c+ cDCs purified from mice treated with fucoidan for 2 hours showed dramatically elevated IL-6, IL-12p40, and TNF-α mRNA levels compared with control mice. Thus, systemic administration of fucoidan induced the maturation of splenic cDCs, as indicated by the upregulation of costimulatory molecules and the production of proinflammatory cytokines.
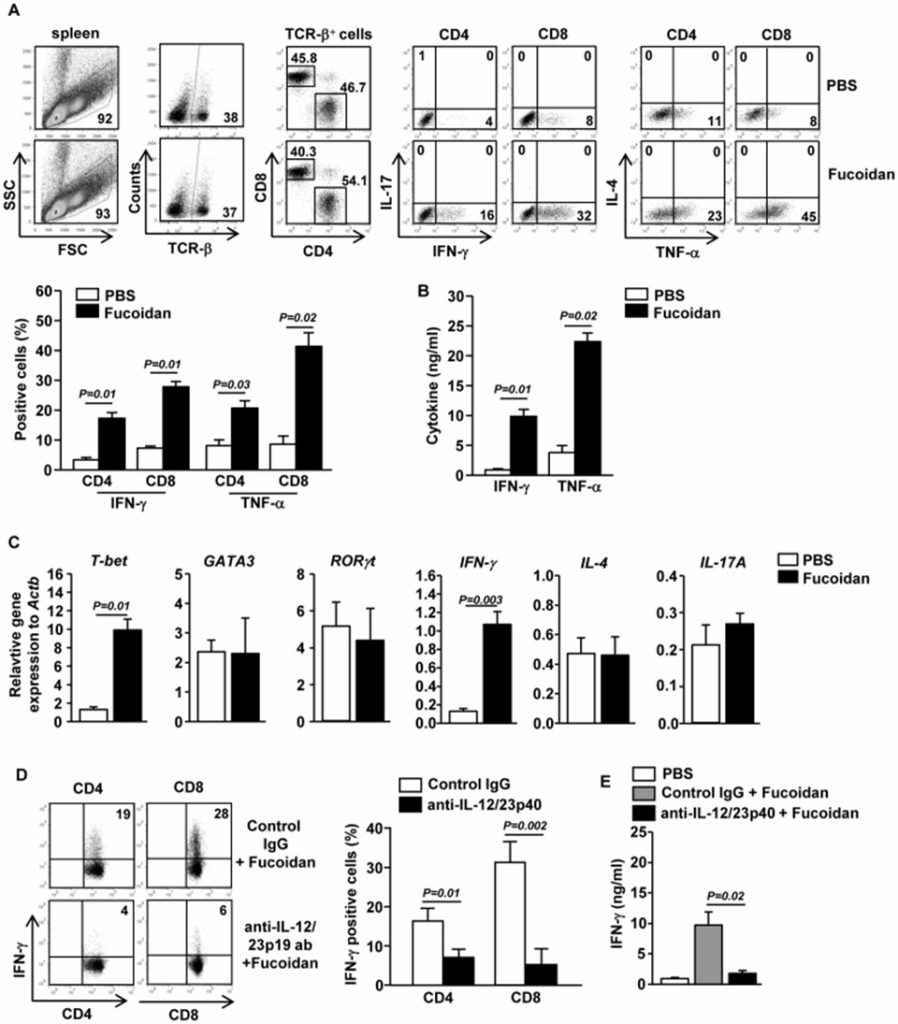
The study examined whether the maturation of splenic cDCs by fucoidan, which it induced in both CD8α+ and CD8α− cDCs, could then stimulate CD4 and CD8 T cell responses in a living system. Mice were intraperitoneally injected with 10 mg/kg of fucoidan, followed by a second injection of the same amount of fucoidan 3 days later. Fucoidan administration significantly increased the percentage of splenic CD4 and CD8 T cells producing IFN-γ and TNF-α, the signature cytokines of Th1 and Tc1 cells (Figure 1A). In comparison, fucoidan did not increase the percentage of splenic CD4 and CD8 T cells producing IL-17 or IL-4 (Figure 1A). In addition, fucoidan treatment resulted in a significant rise in IFN-γ and TNF-α serum levels (Figure 1B). Furthermore, compared to control mice, the mRNA levels of T-bet (p=0.01) and IFN-γ (p=0.003), key transcription factors for Th1 and Tc1 cells, were significantly increased in the spleen of mice treated with fucoidan (Figure. 1C). On the other hand, the mRNA levels of GATA3 and RORγt, transcription factors for Th2 and Th17 cells, were not affected by fucoidan treatment (Figure. 1C).
Next, they examined whether the fucoidan-induced enhancement of Th1 and Tc1 responses was dependent on IL-12, a key inducer of Th1 and Tc1 cells in various immune responses. Following pretreatment with fucoidan or PBS, C57/B6 mice were given an injection of anti-IL-12/23p40 antibody. The fucoidan-induced enhancement of IFN-γ production in CD4 and D8 T cells was almost completely blocked by IL-12/23p40 neutralization (Figure 1D). Furthermore, the fucoidan-induced increase in serum IFN-γ levels was also completely blocked by anti-IL-12/23p40 antibody administration (Figure 1E). Therefore, Fucoidan facilitates the creation of IFN-γ-producing Th1 and Tc1 cells, a process that is IL-12 dependent. These data, combined with the observation that fucoidan enhances IL-12 production by DCs, suggest that fucoidan promotes Th1 and Tc1 responses by enhancing IL-12 production.
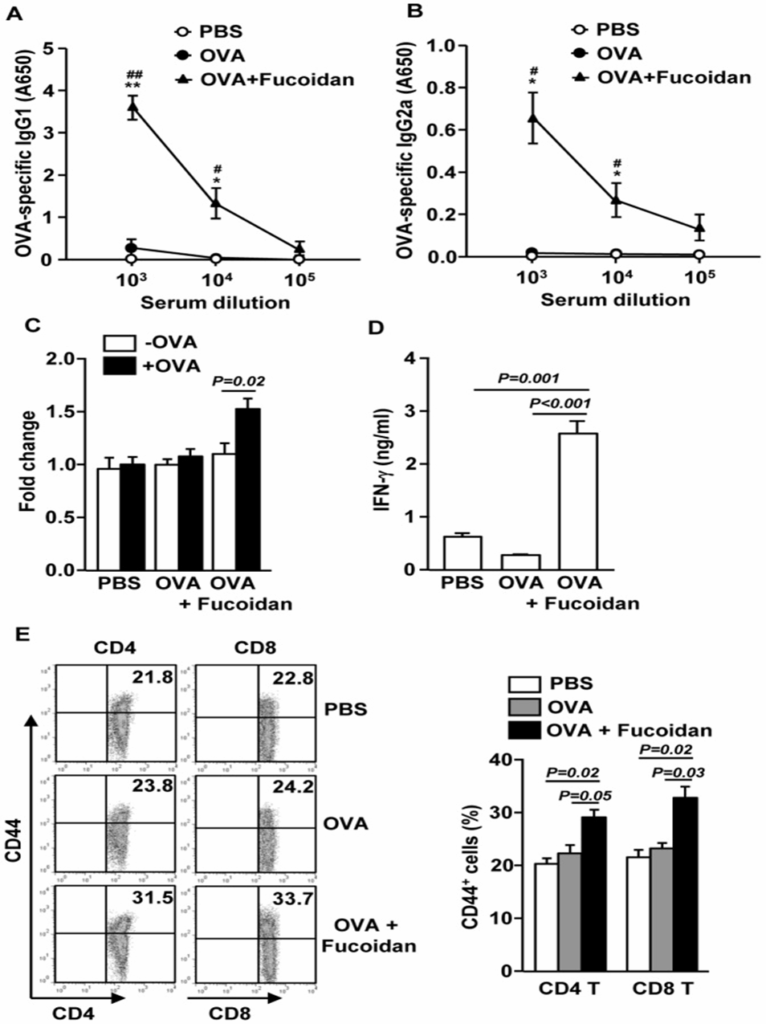
The study investigated whether fucoidan boosts the immune response in living animals by immunizing mice with OVA and fucoidan, and subsequently analyzing the production of OVA-specific antibodies and T cells. Mice immunized with OVA + fucoidan produced significantly more anti-OVA IgG1 and IgG2a than control mice immunized with OVA alone (Figures 2A and B). On day 35, splenocytes were also harvested and analyzed for OVA-induced T cell responses after restimulation with OVA in vitro for 4 days. Splenocytes from mice immunized with OVA + fucoidan showed significantly higher cell proliferation and IFN-γ production than those from control mice immunized with OVA alone (Figures 2C and D).
According to the results, fucoidan could be an adjuvant, possibly by boosting Th-type immune responses. Next, they examined whether fucoidan promoted the generation of effector/memory T cells in OVA-immunized mice based on the surface expression of CD44. As shown in Figure 2E, injection of fucoidan resulted in a significant increase in the percentage of CD44+ CD4 and CD8 T cells (Figure 2E). Based on the data, fucoidan seems to function as an adjuvant, thus improving antigen-specific T and B cell immune responses.
Researchers started their study by investigating whether fucoidan could boost antigen presentation or cross-presentation by dendritic cells, in order to learn more about how it helps stimulate antigen-specific T cell responses within a biological system. Mice were injected with PBS, OVA, or OVA + fucoidan for 24 hours, and the expression of MHC class I and II molecules on splenic Lineage-CD11c + cDCs was measured. Splenic CD11c + cDCs showed dramatically increased surface expression of MHC class I and II molecules after OVA + fucoidan administration, but not after OVA alone.
Following this, they conducted adoptive transfer experiments to observe the expansion of OT-I and OT-II T cells that are specific to OVA. CFSE-labeled OT-I CD8 T cells or OT-II CD4 T cells were transferred into CD45.1 allogeneic mice, and 24 hours later, the mice were injected with PBS, OVA, or OVA + fucoidan. Three days later, the proliferation of OT-I and OT-II cells was measured by a CFSE dilution assay. Mice given OVA plus fucoidan had considerably greater expansion of OT-I and OT-II T cells than mice given only OVA. These data demonstrated that fucoidan functions as an adjuvant to promote antigen presentation and activation of antigen-specific CD4 and CD8 T cells.
Based on the observation that fucoidan functions as an adjuvant to activate OVA-specific CD4 and CD8 T cells, they further investigated whether this response could protect mice implanted with OVA-expressing B16 tumor cells. Mice immunized with OVA + fucoidan were almost completely protected from B16-OVA tumor challenge and did not develop tumors after a second tumor challenge, suggesting the formation of long-term memory. They also investigated CTL functional activity in an in vivo cytotoxicity assay. Mice immunized with OVA + fucoidan showed an 80% lysis rate of specific target cells, suggesting the induction of T cell memory. There was no significant cell death in mice vaccinated with either OVA or fucoidan alone. Taken together, these data suggest that fucoidan may induce OVA cross-presentation by dendritic cells (DCs), resulting in the priming of OVA-specific CTLs that kill target cells in vivo.
To summarize, the findings indicate that fucoidan from Fucus vesiculosus acts as a new adjuvant, boosting dendritic cell maturation, activating CTLs, enhancing Th1 immune responses, generating antigen-specific antibodies, and creating memory T cells. There is hope that fucoidan can be utilized as an adjuvant in vaccines designed to fight tumors.
Source: PLoS One. 2014 Jun 9;9(6):e99396. doi: 10.1371/journal.pone.0099396
