The anti-cancer drug treatment known as chemotherapy can induce a range of side effects, including blood toxicity (bone marrow suppression), gastrointestinal issues such as fever, nausea, vomiting, loss of appetite, constipation, and diarrhea, neurological problems like peripheral neuropathy manifesting as numbness and pain in the hands and feet, allergic responses (hypersensitivity), fatigue, inflammation of the mouth (stomatitis), skin disorders affecting the hands and feet (hand-foot syndrome), and impaired kidney function.
For patients with advanced or recurrent colorectal cancer, combination chemotherapy, specifically FOLFOX or FOLFIRI, is the cornerstone of treatment, offering extended survival with prolonged use. Therefore, controlling the toxicity of these agents could be crucial to extending survival. However, extensive research shows fucoidan, a major sulfated polysaccharide in brown algae, has many biological activities, including anti-inflammatory and anti-tumor effects.
In this blog, I would like to share the following study, “Fucoidan reduces the toxicities of chemotherapy for patients with unresectable advanced or recurrent colorectal cancer” by Masahide Ikeguchi et al.
In a study designed to investigate the impact of Cladosiphon okamuranus (Okinawamozuku) fucoidan on mitigating the toxicity associated with anticancer drugs, a total of 20 participants diagnosed with unresectable, advanced, or recurrent colorectal cancer, and slated for treatment using either FOLFOX or FOLFIRI regimens, were randomly allocated into two distinct groups: a fucoidan treatment group comprising 10 participants, and a control group of equal size (n=10) that did not receive the fucoidan treatment, in order to assess the effectiveness of fucoidan in reducing the adverse effects of chemotherapy.
The absence of side effects, including allergic dermatitis, allowed all 20 participants to finish 6 months of fucoidan treatment. Furthermore, no patients died due to chemotherapy toxicity. Over the study period, 307 cycles of mFOLFOX6 or FOLFIRI were given, with patients receiving an average of 15.4 cycles (between 7 and 38 cycles). The mean number of treatment cycles in the fucoidan group (19.9) was significantly higher than in the control group (10.8 cycles, P = 0.016).
Chemotherapy-related toxicities were monitored, and no patients in either group experienced severe (grade 4) toxicities. The occurrence of diarrhea and neurotoxicity was not suppressed by fucoidan. Myelosuppression was found to be similar in the fucoidan and control groups. In contrast, general fatigue was detected in 60% of the control group but was significantly suppressed to 10% in the fucoidan group.
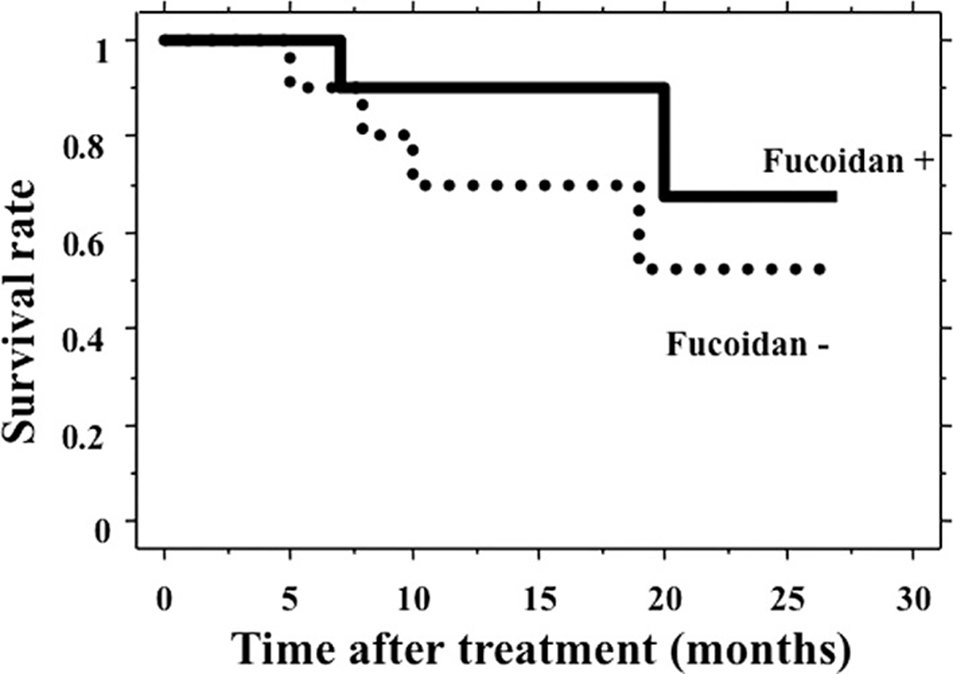
After that, the patients were followed up, and the median follow-up period for the 20 patients was 15 months (range 5-27). Colorectal cancer progression caused the death of six patients during the follow-up; two were in the fucoidan group, and four were in the control group. The survival time of the 10 patients who received fucoidan treatment was longer than that of the 10 patients in the control group, but the difference was not significant (P=0.314, Figure 1).
The observed effect of fucoidan was a significant decrease in the occurrence of fatigue as a side effect of chemotherapy. Chemotherapy with fucoidan was continued for a longer period than chemotherapy without fucoidan. The survival time of patients who received fucoidan treatment was longer than that of patients who did not, but the difference was insignificant. Fucoidan may, however, allow for continuous chemotherapy in patients with unresectable, advanced, or recurrent colorectal cancer, potentially improving their prognosis.
Source: Oncol Lett. 2011 Jan 21;2(2):319–322. doi: 10.3892/ol.2011.254
