In the United States, thyroid cancer accounts for approximately 2% of malignant tumors and is responsible for more than 1,600 cancer-related deaths annually. Thyroid cancer accounts for 1% of human cancer cases and includes well-differentiated thyroid cancer of the papillary and follicular types, which account for more than 95% of thyroid cancer cases, and anaplastic thyroid carcinoma (ATC), a form of thyroid malignancy. ATC is a life-threatening disease with a median survival rate of 6 months after diagnosis. Of patients diagnosed with ATC, 90% will have extra-glandular metastases at the time of diagnosis, and 75% of patients will develop distant metastases.
In this blog, I would like to inform you of the study, “Antitumor activity of fucoidan in anaplastic thyroid cancer via apoptosis and anti-angiogenesis” by Hong‑Yan Shenet et al, which demonstrated the effects of Fucus vesiculosus-derived fucoidan on cell proliferation and apoptosis in anaplastic thyroid carcinoma cells.
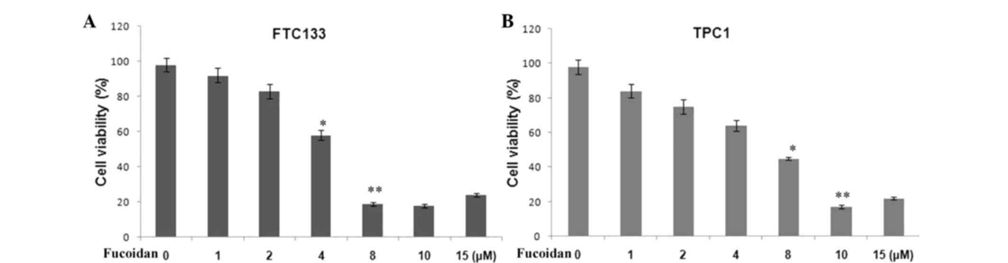
First, treatment of thyroid cancer (FTC133) and (TPC1 ATC) cell lines with different concentrations of fucoidan (1–15 µM) inhibited cell proliferation 36 hours after treatment. The inhibition of cell proliferation by fucoidan was observed in a concentration-dependent manner (Figure 1). The half-maximal inhibitory concentrations for proliferation inhibition in FTC133 and TPC1 cell lines were 8µM and 10µM, respectively.
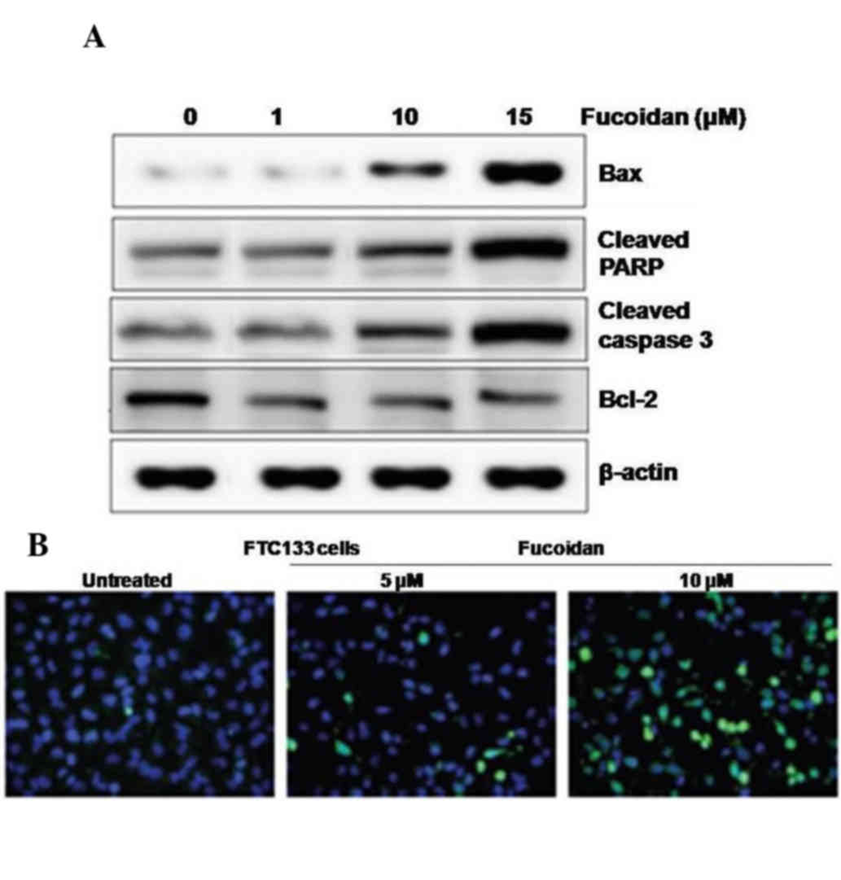
Western blot analysis showed that the effect of fucoidan on apoptotic cell death in FTC133 ATC cells was increased expression of Bax, cleaved PARP, and caspase-3, and decreased expression of Bcl-2 in FTC133 cells after 36 h of fucoidan treatment compared to control cells (Fig. 2A). DAPI staining revealed DNA fragmentation and the appearance of apoptotic bodies at the nuclear periphery in 8 µM fucoidan-treated FTC133 cells (Fig. 2B). Fucoidan induced apoptosis in FTC133 cells. This was indicated by DNA strand breaks and was also observed by TUNEL staining.
Next, to investigate the effect of fucoidan on angiogenesis, cells were treated with CoCl2 (100 µM) for 18 h to induce hypoxia and then treated with different doses of fucoidan. Hypoxia promoted HIF-1α expression, but fucoidan (10 µM) treatment suppressed hypoxia-induced HIF-1α expression. VEGF expression was also promoted under hypoxia, and this hypoxia-induced VEGF expression was suppressed by fucoidan treatment. Tube formation assay clearly showed that the formation of vessel-like structures was suppressed after fucoidan treatment. Wound cells were cultured for 16 h in a medium containing 50 ng/ml VEGF and 1 mM thymidine with fucoidan (10 µM), showing a loss of wound healing ability. The effect of fucoidan on VEGF expression was examined, and it was found that fucoidan reduced VEGF expression under hypoxic conditions. Thus, fucoidan inhibited tube formation and cell migration, suggesting that fucoidan has a strong anti-angiogenic effect.
In the study, TUNEL and DAPI staining were used to investigate the apoptotic effect of fucoidan. The results demonstrated that fucoidan promoted the expression levels of cleaved caspase 3 and PARP, and induced apoptosis in thyroid cells. Anti-apoptotic regulators such as Bcl-2 and pro-apoptotic regulators such as Bax are involved in the maintenance of cellular homeostasis. Fucoidan was shown to enhance Bax expression and decrease Bcl-2 expression in FTC133 human thyroid cells, and also suppressed the expression levels of HIF-1α and VEGF under CoCl2-induced hypoxic conditions in FTC133 cells. The antiangiogenic effect of fucoidan was supported by the observed inhibition of cell migration and tube formation, suggesting that fucoidan not only directly targets vascular endothelial cells but also inhibits angiogenesis via VEGF. Fucoidan has great potential as an anticancer drug, and in conclusion, the study has demonstrated the anticancer effects of fucoidan in ATC cells, namely, inducing apoptosis and inhibiting angiogenesis by inhibiting VEGF expression via suppression of HIF-1α.
Source: 15(5):2620-2624. doi: 10.3892/mmr. 2017.6338. Epub 2017 Mar 16.
