Morbidity and mortality associated with current treatments for acute promyelocytic leukemia (APL) are high, and despite improvements in patient survival, acute myeloid leukemia is often rapidly progressive, leading to the sudden onset of symptoms. Prompt diagnosis and initiation of treatment are key but remain major clinical concerns. Therefore, developing adjuvant therapies that increase efficacy while reducing morbidity is crucial.
On the other hand, Fucoidan has antitumor and immunomodulatory effects and low toxicity, making it potentially beneficial as an adjunctive therapy.
In this blog, I would like to inform you of the study, “Fucoidan enhances the therapeutic potential of arsenic trioxide and all-trans retinoic acid in acute promyelocytic leukemia, in vitro and in vivo” by Farzaneh Atashrazm et al, which showed synergistic effects of the antitumor agent fucoidan with current APL treatments.
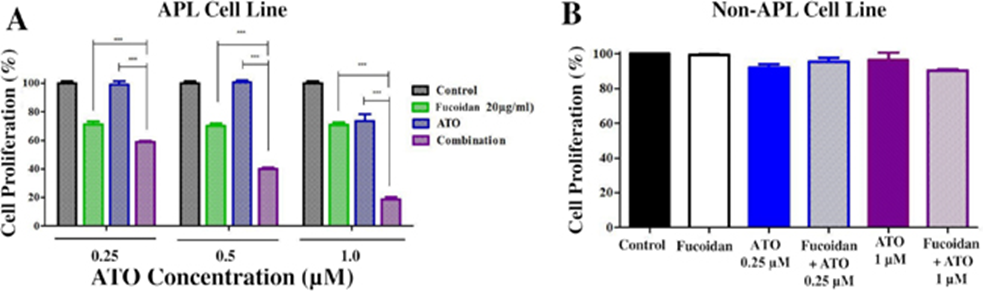
First, the acute promyelocytic leukemia NB4 cell line was treated with 20 μg/ml fucoidan, and increasing concentrations of arsenic trioxide (ATO) and cell proliferation were assessed. NB4 cell proliferation was significantly reduced by combined treatment with fucoidan and ATO compared to treatment with either agent alone (Figure 1A). To examine whether the observed ATO activity was specific to APL cells, the Kasumi-1 AML(acute myeloid leukemia) cell line was similarly treated with ATO and fucoidan. The ATO/fucoidan combination, or fucoidan and ATO alone, did not reduce Kasumi-1 cell proliferation, and the percentage of viable cells in all groups remained above 80% up to 96 hours (Figure 1B).
Next, a propidium iodide assay was performed to examine the effect of the combination of fucoidan and ATO on the cell cycle. Treatment of cells with fucoidan + ATO significantly increased the sub-G0/G1 population, representing dead cells, compared to low and therapeutic doses of ATO alone. The combination of ATO and fucoidan reduced the G0/G1, S, and mitotic phase populations compared to ATO alone.
To determine whether cell death was due to apoptosis, we employed the Annexin V/PI assay. Low doses of ATO increased the percentage of apoptotic cells (Annexin V-positive cells). A therapeutic dose of 1 μM ATO increased the percentage of apoptotic cells from approximately 18% (ATO only) to 71% (fucoidan + ATO).
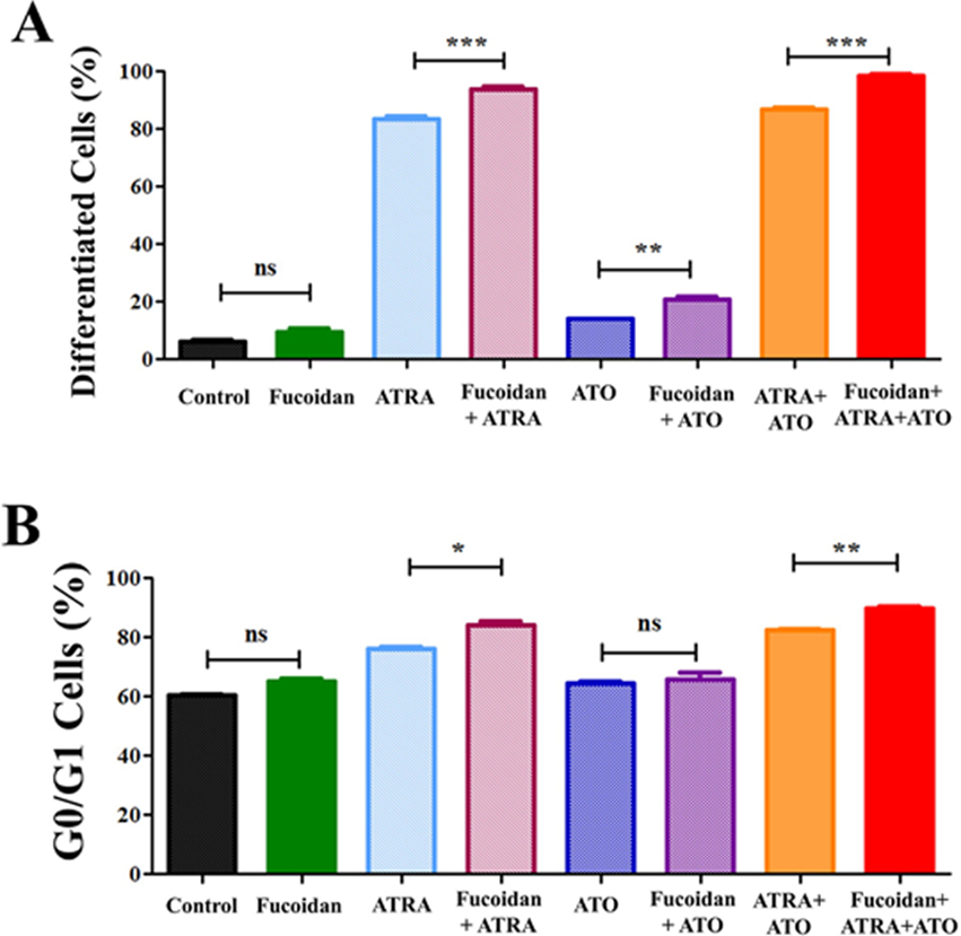
NB4 cells were treated with fucoidan, low-dose all-trans retinoic acid (ATRA), or ATO, either alone or in combination. A very low dose of fucoidan (5 μg/ml) was chosen because fucoidan has a cytotoxic effect on NB4 cells. Myeloid differentiation was then measured by measuring the expression level of CD11b (identified by mean fluorescence intensity). Differentiated cells were identified by CD11bhigh expression. When NB4 cells were co-cultured with fucoidan and ATRA, ATO, or ATRA+ATO, there was an increase in CD11bhigh cells compared to ATRA.
ATO, or ATRA+ATO alone. Treatment with 0.5 μM ATRA alone increased differentiated cells from approximately 6% to 83%, whereas the addition of fucoidan to ATRA increased differentiated cells to 94%. Low-dose ATO similarly increased differentiated cells from 6% to 14%, while fucoidan + ATO increased the percentage of differentiated cells to 20%. Finally, APL cells treated with the combination of the three reagents increased to 99.5% differentiated cells after a 5-day culture period, compared to 87% when cells were treated with ATRA + ATO (Figure 2A).
Because the dose of fucoidan used was very low, the amount of sub-G0/G1 dead cell population did not change when comparing treated and untreated cells. Because differentiating cells exit the cell cycle during mitosis, we analyzed G0/G1 arrest and compared G0/G1 populations within treated groups. Consistent with the CD11b expression data, we observed a significant increase in the G0/G1 population in ATRA+fucoidan co-treated cells compared to ATRA alone, and in ATRA+ATO+fucoidan co-treated cells compared to ATRA+ATO (Figure 2B).
To evaluate the in vivo synergy of these drugs, xenograft APL masses were created subcutaneously in nude mice, and the effect of the drugs on tumor regression was evaluated.
Of the seven mice treated with fucoidan + ATO, two completed the 28-day treatment. All mice in the other groups failed to complete the 28-day treatment. Treatment with “fucoidan only” and “fucoidan + ATO” significantly inhibited tumor mass development, and tumors in these animals reached their maximum endpoint in a longer period than in the control group. Despite the higher median survival time in the combination group, the difference between the treatment groups was not statistically significant. Tumor aggressiveness, as assessed by tumor volume, was reduced in the treatment groups compared to the control group.
In the analysis of the antitumor effect of ATRA + fucoidan, one animal in the fucoidan alone group and one animal in the ATRA alone group completed 28 days of treatment. Significant regression of APL tumors was observed in all three treatment groups compared to the control group.
At the end of the in vivo study on the combined effect of fucoidan and ATRA, tumor masses were removed, and NB4 cell differentiation was evaluated by CD11b expression. Tumor cells were identified by CD44 expression, as nearly all NB4 cells highly express CD44. A significant increase in differentiation was observed in tumor masses treated with fucoidan alone and fucoidan + ATRA, but there was no significant difference between the treatment groups.
Furthermore, distinct immunophenotypic patterns were observed in the expression of CD44 and CD11b in the different treatment groups. The expression of CD44 on differentiated cells was evaluated, and the ratio of CD11bhighCD44- cells to CD11bhighCD44+ cells was calculated. The ratios were 0.58, 0.83, 0.87, and 1.14 in the control, fucoidan-only, ATRA-only, and combination groups, respectively, indicating that CD44 expression was decreased in mice treated with fucoidan + ATRA.
As a result, this leads to decreased cell migration in patients with APL. Our findings provide evidence supporting the use of fucoidan as an adjunct therapeutic agent in the treatment of APL.
Source: Oncotarget. 2016; 7:46028-46041. https://doi.org/10.18632/oncotarget.10016
