Cisplatin has become a cornerstone in the treatment of numerous types of cancer, serving as a standard and frequently utilized chemotherapeutic agent. On the other hand, severe ototoxicity is a known side effect of cisplatin treatment. On the other hand, fucoidan, a complex sulfated polysaccharide primarily derived from brown algae, exhibits diverse physiological activities, including antibacterial, anti-inflammatory, anticancer, and antioxidant properties. Research on the otoprotective effects of fucoidan via its antioxidant properties is limited. This blog post aims to share information regarding a significant study conducted by Cheng-Yu Hsieh and his colleagues, titled “Otoprotective Effects of Fucoidan Reduce Cisplatin-Induced Ototoxicity in Mouse Cochlear UB/OC-2 Cells”. The study examined fucoidan’s capacity to safeguard UB/OC-2 mouse cochlear cells against cisplatin-induced ototoxicity in an in vitro model, potentially leading to a new treatment method.
First, to investigate the cytotoxicity of cisplatin by using mouse cochlear UB/OC-2 cells, we first assessed cell viability using a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Cisplatin significantly inhibited cell viability in a dose-dependent manner. Because UB/OC-2 cell viability decreased to approximately 50% after treatment with 5 μM cisplatin, we used 5 μM cisplatin in subsequent experiments. Additional research was conducted into the cytotoxicity of fucoidan within mouse cochlear UB/OC-2 cells. Treatment with fucoidan alone at concentrations up to 2 mg/mL had little to no effect on cell viability. The subsequent experiments will utilize 2 mg/mL of fucoidan, as determined by these findings.
To evaluate the effect of fucoidan on cisplatin-treated UB/OC-2 cells, cells were treated with fucoidan, and cytotoxicity was measured based on cell viability. Compared with the control group, cytotoxicity was significantly increased in the cisplatin group and decreased in the fucoidan-treated group. Fucoidan’s protective effect against cisplatin-induced cytotoxicity in mouse cochlear UB/OC-2 cells is suggested by this finding.
Cisplatin-induced ROS production is associated with mitochondrial dysfunction and can induce apoptosis via a decrease in mitochondrial membrane potential. As a result, intracellular ROS generation, assessed with the fluorescent dye 2′,7′-dichlorodihydrofluorescein diacetate (DCFDA), was substantially elevated in the cisplatin group and significantly reduced in the fucoidan-treated group. To further evaluate the effect of fucoidan on cisplatin-induced oxidative damage, mitochondrial membrane potential was analyzed using the JC-1 green/red fluorescence intensity ratio. When compared to the control group, there was an elevation in this ratio for the cisplatin group and a substantial decline for the fucoidan group. The mitochondrial apoptotic pathway leads to the outer mitochondrial membrane becoming permeable, which in turn causes cytochrome C to be released from the mitochondria into the cytoplasm. Fucoidan treatment lessened the accumulation of cytochrome C in the cytoplasm caused by cisplatin exposure. The findings suggest that fucoidan may inhibit the intracellular production of reactive oxygen species (ROS) and mitochondrial dysfunction caused by cisplatin.
The expression levels of the pro-apoptotic protein Bax and the anti-apoptotic protein Bcl-2 were analyzed to elucidate the role of fucoidan in the mitochondrial apoptotic pathway. Fucoidan administration increased Bcl-2 expression at both the mRNA and protein levels, while Bax expression decreased. In order to clarify the intrinsic apoptotic pathway involved in cisplatin-induced cell death, the reactive expression of apoptosis-related proteins was assessed via Western blot analysis. In the cisplatin group, there was a notable and significant elevation in the expression levels of cleaved caspase 9, cleaved caspase 3, and cleaved poly(ADP-ribose) polymerase (PARP), whereas in the fucoidan group, a reduction in these same markers was observed. These results suggest that fucoidan attenuates cisplatin-induced apoptosis in mouse cochlear UB/OC-2 cells.
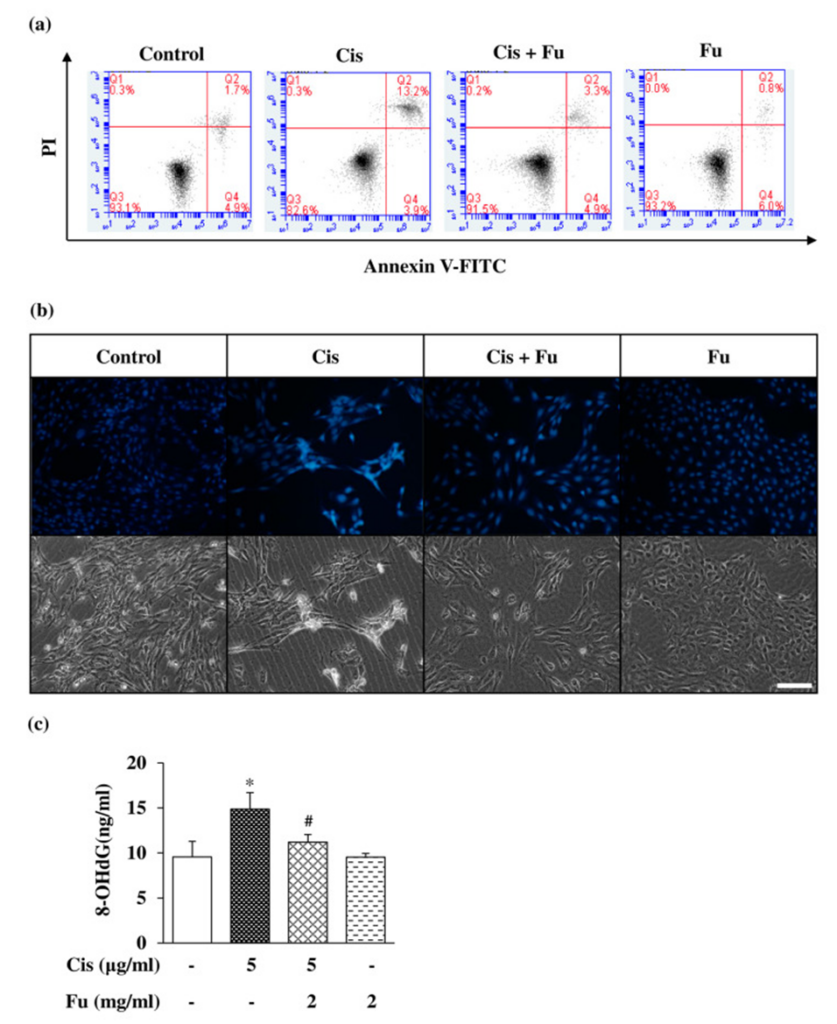
The next step involved studying fucoidan’s role in cisplatin-induced apoptosis and quantifying intracellular cellular damage. For this assessment, they utilized flow cytometry, incorporating both Annexin V/PI double staining and Hoechst 33258 staining, a chromatin-targeting dye. It was observed that the number of cells undergoing apoptosis was markedly higher in the group treated with cisplatin when contrasted with the control group, while the fucoidan-treated group exhibited a reduction in these values, as depicted in Figure 1a.
Hoechst 33258 staining was performed to evaluate cell morphology and nuclear condensation in apoptotic cells. The study observed a substantial rise in bright blue fluorescence within apoptotic cells, contrasting with the control group (see Figure 1b). The study examined the capacity of fucoidan to decrease oxidative stress resulting from cisplatin. The 8-hydroxydeoxyguanosine (8-OHdG) assay revealed a significant reduction in oxidative DNA damage when fucoidan was administered, in contrast to the effects observed with cisplatin, as depicted in Figure 1c. These results suggest that fucoidan reduces cisplatin-induced apoptotic cell death.
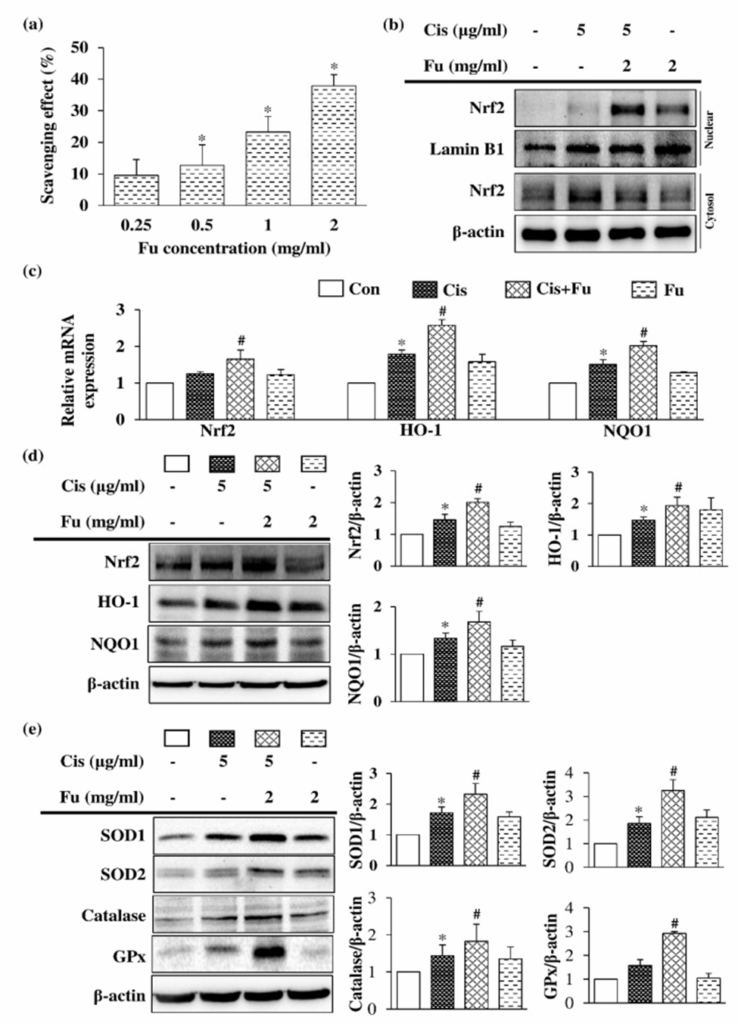
Fucoidan exerts its antioxidant effects through its free radical scavenging activity in a dose-dependent manner as seen in Figure 2a. Under normal physiological conditions, nuclear factor erythroid 2-related factor 2 (Nrf2) binds to Kelch-like ECH-associated protein 1 (Keap1) and is destabilized in the cytoplasm. However, under oxidative stress conditions, the binding of Keap1 to DLG is inhibited, allowing Nrf2 to rapidly translocate and accumulate in the nucleus, thereby activating the transcription of downstream antioxidant enzyme genes. Figure 2b shows that the administration of fucoidan led to a considerable rise in the protein expression level of Nrf2 within the nuclear fraction.
Figure 5c illustrates that treating UB/OC-2 cells with different concentrations of fucoidan under cisplatin-induced oxidative stress resulted in a significant elevation of Nrf2 mRNA levels, along with the mRNA expression of downstream enzymes Ho1 and Nqo1. Under conditions of oxidative stress, fucoidan treatment of UB/OC-2 cells resulted in a significant elevation of protein expression for Nrf2, HO-1, and NQO1, as seen in Figure 2d. In addition to these findings, fucoidan administration resulted in a significant upregulation of protein expression for other antioxidant enzymes, including SOD1, SOD2, catalase, and GPx, relative to the cisplatin group, see Figure 2e. The combined results lead to the conclusion that fucoidan may exert its antioxidant effects on oxidative stress via NRF2-governed antioxidant enzymes. The study’s findings proved that administering fucoidan resulted in diminished cisplatin-induced intracellular reactive oxygen species, a stable mitochondrial membrane potential, controlled mitochondrial dysfunction, and robust protection of hair cells from apoptosis. Fucoidan also worked to counteract oxidative stress by modulating the NRF2 pathway, thereby exhibiting antioxidant effects. Fucoidan thus presents itself as a possible therapeutic compound advancing novel otoprotective approaches.
Source: Int J Mol Sci. 2023 Feb 10;24(4):3561. doi:10.3390/ijms24043561
