Hepatocellular carcinoma, which is identified as a primary and significant form of liver cancer, stands out as one of the most fatal types of tumors, primarily attributed to its substantial morbidity rates and generally unfavorable prognosis. On the other hand, Fucoidan has been demonstrated to exhibit a broad spectrum of biological activities, encompassing antioxidant, anti-inflammatory, and antitumor properties.
This blog post aims to share a significant study by Danhui Ma et al., “Fucoidan Inhibits the Progression of Hepatocellular Carcinoma via Causing lncRNA LINC00261 Overexpression“. The study delves into how fucoidan effectively inhibits the advancement of hepatocellular carcinoma by leading to the overexpression of lncRNA LINC00261, and further elaborates on fucoidan’s capacity to hinder cell proliferation in both in vivo and in vitro environments, impede cell motility and invasion, and instigate cell cycle arrest along with apoptosis.
First, to verify whether fucoidan can inhibit HCC proliferation, MHCC-97H cells were treated with saline (Ctrl), 0.25, and 0.5 mg/mL fucoidan (Fuc) for 48 hours. Compared to the control group, fucoidan significantly inhibited MHCC-97H cell proliferation in a concentration-dependent manner, and morphological changes suggestive of cell apoptosis, such as budding and vacuolization, were observed. To further confirm this observation, cell proliferation experiments were performed. Equal numbers of cells were treated with saline, 0.25 mg/mL, or 0.5 mg/mL fucoidan for 48 hours. Results showed that the cell proliferation ability decreased after fucoidan treatment, and the inhibitory effect was positively correlated with the dose. When Hep3B cells were used in further experiments, similar results were observed.
In addition, cell viability assays were conducted after exposing both MHCC-97H and Hep3B cells to saline or fucoidan for 24, 48, and 72-hour intervals. The results suggested that fucoidan inhibited cell viability, with the inhibitory effect occurring within 48 hours. Clone formation results further confirmed the inhibitory effect of fucoidan on HCC cell proliferation compared to the control group.
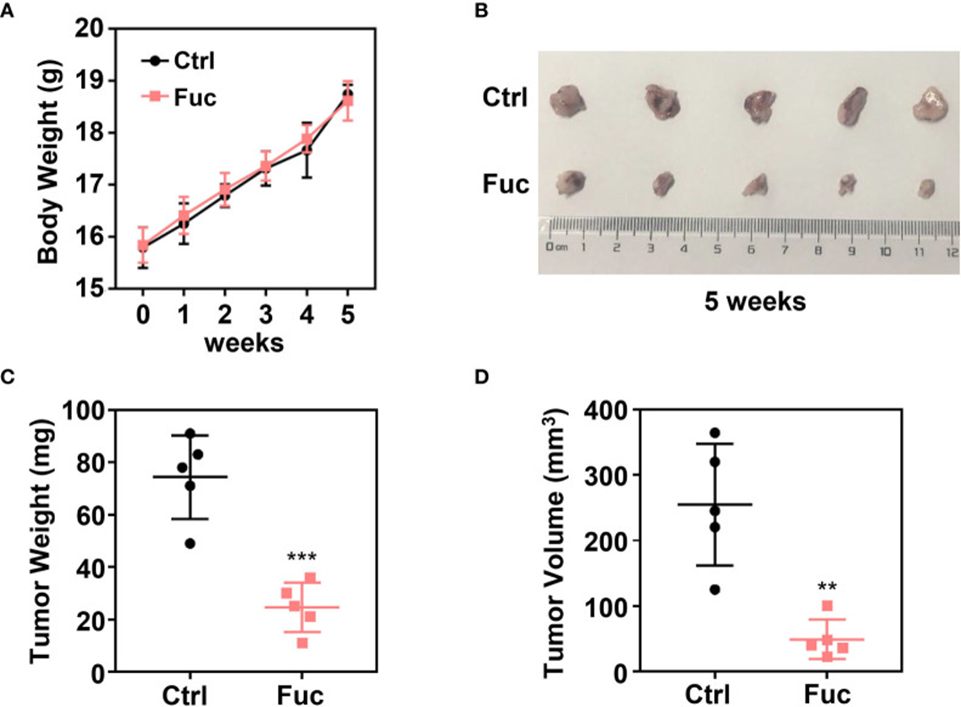
Next, to investigate whether fucoidan inhibits HCC cell growth in vivo, a xenograft tumor model was established. MHCC-97H cells were subcutaneously injected into 4-week-old female Balb/c nude mice. Two weeks later, 15 mg/kg of fucoidan was orally administered daily for three weeks. Mice were weighed weekly, and tumor weight and volume were recorded at the end of treatment. The data implied that fucoidan posed little danger to mice (see Figure 1A) and was effective in significantly shrinking tumors in terms of both weight and volume in vivo (see Figures 1B-D).
A wound healing assay was conducted to determine if fucoidan influences HCC cell motility. The wound width was notably smaller in the control group than in the fucoidan-treated group; however, the 0.5 mg/mL fucoidan treatment yielded better outcomes than the 0.25 mg/mL treatment, implying that fucoidan impedes cell motility, and this impact escalates with increased dosage. Additionally, a transwell assay was performed to examine the effect of fucoidan on the invasive ability of HCC cells. The findings indicated a decrease in cell penetration through the membrane among those treated with fucoidan, which supports the conclusion that fucoidan diminished the invasive capacity of HCC cells. The preliminary findings suggest that fucoidan has an inhibitory effect on the proliferation, motility, and invasion of HCC cells.
Flow cytometry was utilized to investigate the influence of fucoidan on the cell cycle distribution and apoptosis rate of MHCC-97H cells, aiming to further elucidate fucoidan’s role in HCC development. Similarly, cells were seeded in media containing saline, 0.25 mg/mL, and 0.5 mg/mL fucoidan for 48 hours. Treatment of MHCC-97H cells with higher doses of fucoidan increased the S-phase distribution, showing that fucoidan can induce cell cycle arrest at S-phase in a dose-dependent manner. Meanwhile, a higher apoptosis rate was observed in the fucoidan-treated group compared with the control group, which was positively correlated with the fucoidan concentration. The results further imply that fucoidan aids in the induction of apoptosis within HCC cells. Thus, our data illustrate that fucoidan can stop the cell cycle and trigger apoptosis within HCC cells.
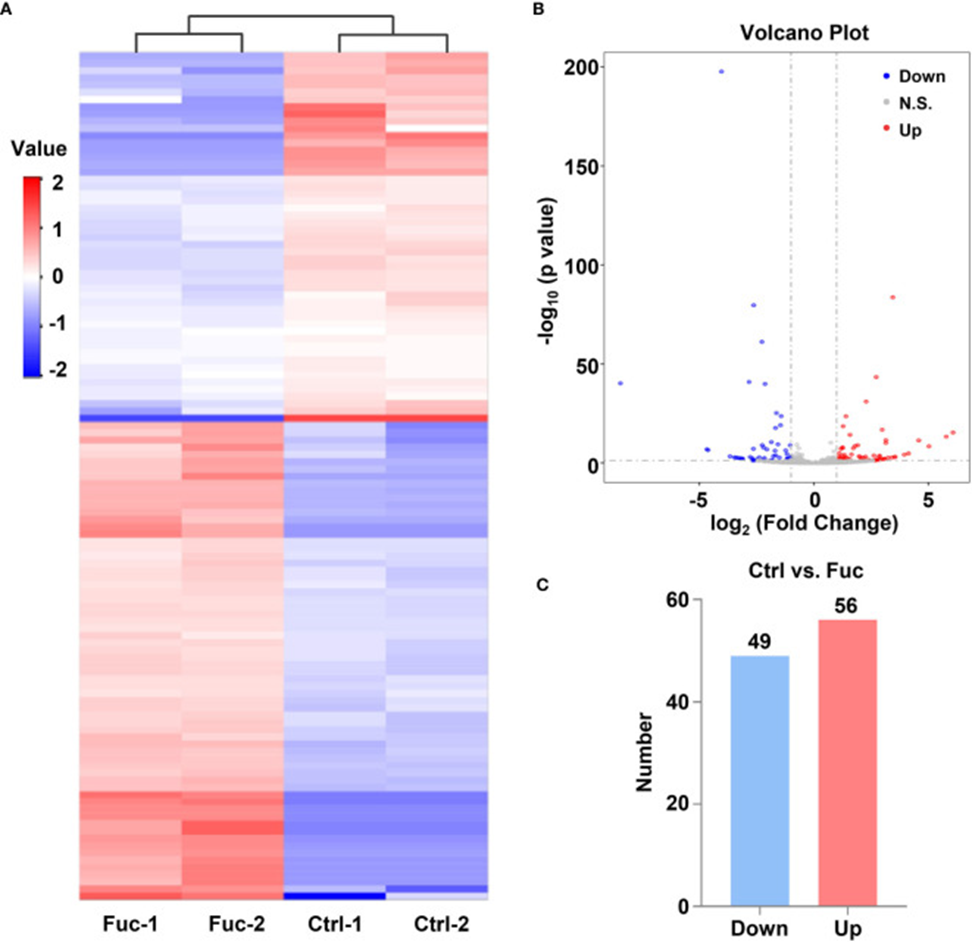
The mechanism through which fucoidan inhibits HCC development was investigated by performing high-throughput sequencing of lncRNAs in MHCC-97H cells exposed to 0.5 mg/mL fucoidan for 48 hours. Analysis of the heatmap results visually confirmed that a large number of lncRNAs were significantly altered. Approximately 75% of the detected lncRNAs were previously annotated, while the remaining 25% were novel (see Figure 2A). A total of 105 differentially expressed lncRNAs (DElncRNAs) were identified using a threshold of log2 (Fold Change) > 1 and adjusted P < 0.05, of which 49 were down-regulated and 56 were up-regulated, as indicated by Volcano Plot results (see Figure 2B, C). To gain a deeper understanding of the mechanisms underlying these DElncRNAs in HCC tumorigenesis, we performed mRNA sequencing. The results showed that 1,633 mRNAs were down-regulated, and 1,737 mRNAs were up-regulated. Analysis of KEGG pathways further revealed a close association between downstream mRNAs regulated by lncRNAs and HCC. The application of 0.5 mg/mL of fucoidan to MHCC-97H cells led to notable modifications in apoptosis-associated genes.
The researchers chose LINC00261 to investigate whether these various lncRNAs have an impact on the progression of liver cancer tumors. Other studies have confirmed that LINC00261 acts as a tumor suppressor gene in prostate cancer, breast cancer, pancreatic cancer, and many other types of cancer. To illustrate, the expression of downstream miR-1269a was inhibited by LINC00261, leading to the suppression of lung cancer cells. Sequencing data showed a significant surge in LINC00261 levels in the fucoidan group. As there have been few studies on LINC00261’s antitumor effects in HCC, it was selected for additional investigation.
To investigate the role of LINC00261 in HCC tumorigenesis, si-LINC00261 (si-LINC00261-1, si-LINC00261-2, si-LINC00261-3) was transfected into MHCC-97H cells. Scrambled siRNA was used as a negative control. qPCR was then used to detect the relative mRNA expression levels of LINC00261 in MHCC-97H cells. Results showed that LINC00261 expression levels decreased after transfection with the three siRNAs, with si-LINC00261-2 showing the most pronounced knockdown effect. si-LINC00261-2 was then selected for follow-up experiments. To demonstrate the effects of LINC00261 on cell proliferation and viability, cell proliferation and viability assays were performed after transfection with si-LINC00261-2.
The proliferation rate of MHCC-97H cells was notably higher after LINC00261 was knocked down, relative to the negative control group. CCK-8 experiments also demonstrated that transfection of si-LINC00261-2 improved HCC cell viability. Furthermore, a wound-healing assay was performed on MHCC-97H cells transfected with scrambled siRNA and si-LINC00261-2 to examine the effect of LINC00261 on HCC cell motility. The results indicated that LINC00261 also inhibited HCC cell motility. The study concluded from these outcomes that fucoidan can increase LINC00261 expression, which in turn inhibits HCC cell proliferation, viability, and motility, demonstrating antitumor activity.
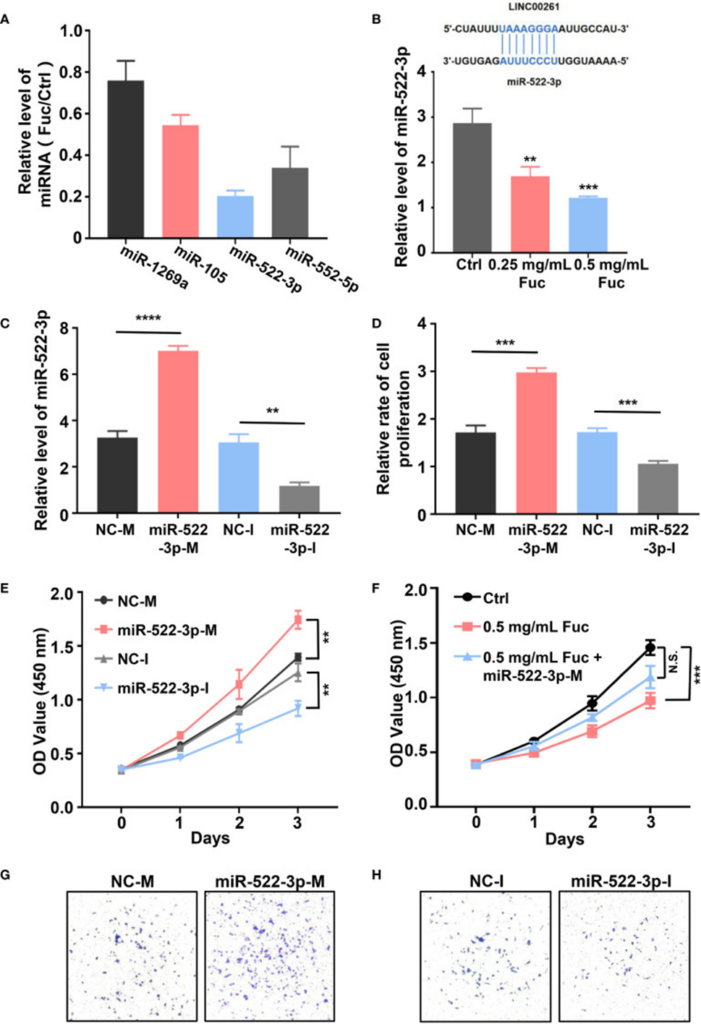
To determine if LINC00261 interacts with miRNAs in MHCC-97H cells, the researchers assessed the expression of miR-1296a, miR-105, miR-522-3p, and miR-552-5p (55, 57–59). These specific miRNAs have previously been documented to bind to LINC00261. qPCR results indicated that miR-522-3p was significantly reduced in the fucoidan-treated group compared with the control group (see Figure 3A). Next, they added equal volumes of saline, 0.25 mg/mL fucoidan, and 0.5 mg/mL fucoidan to MHCC-97H cells, and examined the expression levels of miR-522-3p by qPCR. The results showed that fucoidan downregulated miR-522-3p in a dose-dependent manner (see Figure 3B). Synthesized miR-522-3p mimics and inhibitors from Gene-Pharma were separately introduced into MHCC-97H cells (see Figure 3C). The results suggested that miR-522-3p mimics increased the proliferation rate of MHCC-97H cells, while miR-522-3p inhibitors decreased it (see Figure 3D).
Additionally, the research team assessed cell viability with a CCK-8 assay. They observed that miR-522-3p mimics enhanced the viability of HCC cells, whereas miR-522-3p inhibitors reduced it (see Figure 3E). To further explore the mechanism by which fucoidan inhibits HCC cell viability by regulating miR-522-3p, they transfected MHCC-97H cells with miR-522-3p mimics and simultaneously exposed them to 0.5 mg/mL fucoidan. In comparison to the group administered only 0.5 mg/mL fucoidan, miR-522-3p demonstrated an effective reversal of the fucoidan-triggered suppression of cell viability (see Figure 3F). The Transwell assay results additionally indicated that an increase in miR-522-3p levels stimulated HCC cell invasion, while a decrease in miR-522-3p levels suppressed HCC cell invasion (see Figure 3G, H).In conclusion, fucoidan can upregulate the expression level of LINC00261, which inhibits HCC cell proliferation and invasion through downregulation of miR-522-3p.
Furthermore, fucoidan demonstrably boosted SFRP2 expression in a dose-dependent way, indicating that it elevates miR-522-3p via LINC00261, consequently enhancing SFRP2 levels. The protein level of SFRP2 was notably elevated by fucoidan, which was confirmed through Western blot analysis. The findings indicated that fucoidan inhibits the advancement of hepatocellular carcinoma by promoting increased expression of the lncRNA LINC00261.
Source: Front Oncol. 2021 Apr 13;11:653902. doi: 10.3389/fonc.2021.653902
