Among patients diagnosed with chronic kidney disease (CKD), a frequently encountered complication is the presence of mineral and bone disorder (MBD), which is a recognized consequence of this condition. The need for advanced CKD-MBD treatments is therefore highly significant from a clinical standpoint. Fucoidan (FPS), which originates from the Japanese kelp (Laminaria japonica), is a natural compound that has been found to be beneficial for CKD-MBD. It remains unclear whether FPS can improve CKD-MBD. This blog post will discuss the study “Fucoidan Ameliorates Renal Injury-Related Calcium-Phosphorus Metabolic Disorder and Bone Abnormality in the CKD–MBD Model Rats by Targeting FGF23-Klotho Signaling Axis” by Bu-Hui Liu and et al. This research intends to elucidate how FPS impacts therapeutic outcomes and its underlying mechanisms in both living rats with CKD-MBD and in laboratory settings, contrasting its effects with calcitriol (CTR).
The male rats were first assigned to one of four experimental groups: sham, CKD-MBD, FPS, and CTR. The CKD-MBD rat model was induced by Adenine administration and unilateral nephrectomy. After induction of renal injury, rats were administered FPS, CTR, or vehicle for 21 days. Researchers examined changes in indicators of impaired kidney function, damage to the renal tubules and interstitium, imbalances in calcium and phosphorus metabolism, and bone pathologies.
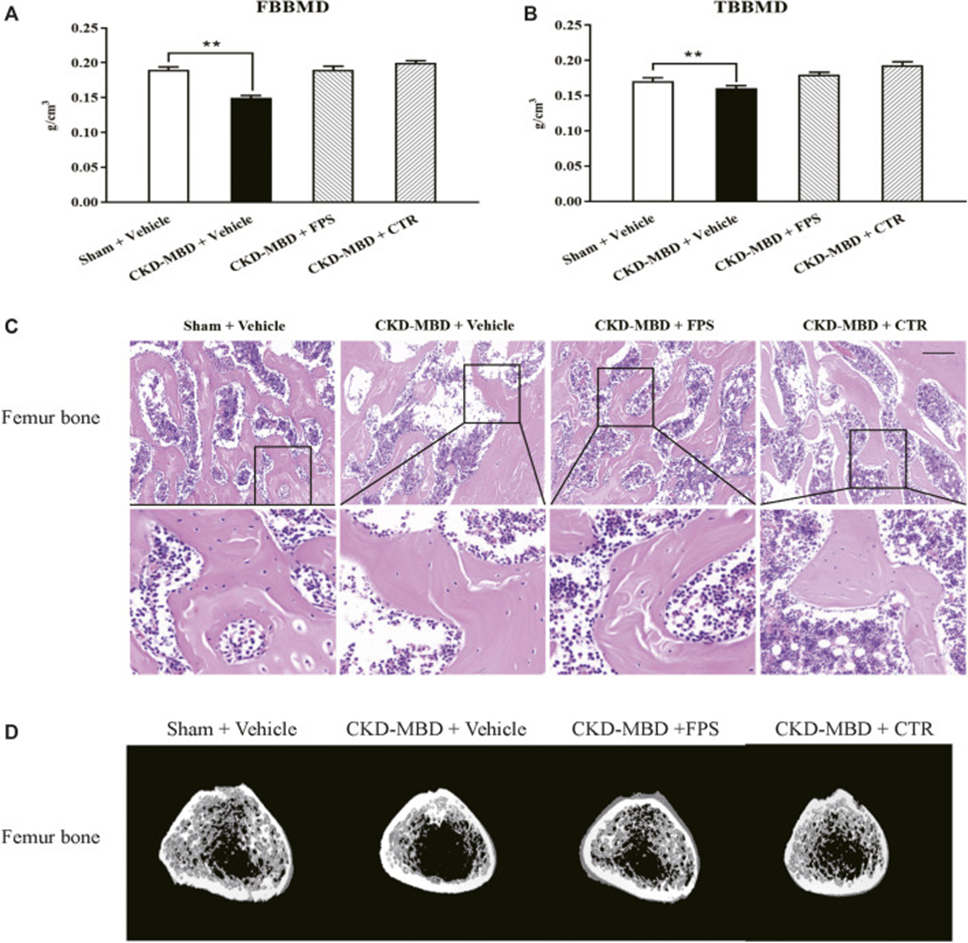
Compared to shame-operated rats, CKD-MBD model rats showed significant changes in urinary Ca2+ and P4+, serum Ca2+, P4+, ALP, VD3, FGF23, and iPTH, as well as significant decreases in TBBMD and FBBMD. Furthermore, HE staining clearly revealed typical bone lesions with resorption cavities in the cortical bone in CKD-MBD model rats 3 weeks after induction of renal injury. The femoral lesions observed in CKD-MBD model rats were marked by greater osteoid and resorption cavities within the cortical bone, an increase in both osteoclasts and osteoblasts, and the presence of fibrosis. These results suggest that both renal injury and bone abnormalities in CKD-MBD model rats are significantly expressed in vivo.
In studies conducted within living organisms, fucoidan (FPS) and calcitriol (CTR) were investigated for their impact on kidney dysfunction and tubulointerstitial damage. The findings indicated that rats subjected to a sham treatment, three weeks after renal damage was induced, exhibited a clear and undamaged renal interstitium along with unobstructed tubules. The CKD-MBD rats, much like the sham-treated rats, demonstrated significant damage to the renal tubulointerstitium. This damage was characterized by the accumulation of extracellular matrix (ECM) and collagen deposition within this specific region. The tubulointerstitial region of FPS and CTR rats showed a significant improvement in ECM accumulation and collagen deposition after three weeks of FPS or CTR administration, when compared to CKD-MBD rats. The given results imply that renal dysfunction and tubulointerstitial damage in CKD-MBD model rats can be mitigated in vivo through the use of FPS and CTR.
The effects of FPS and CTR on urinary Ca2+, P4+, serum Ca2+, P4+, VD3, FGF23, and bone mineral density were evaluated in CKD-MBD model rats. As shown in Figures 3 and 4, after induction of renal injury, CKD-MBD model rats exhibited increased urinary Ca2+, serum P4+, and FGF23, decreased urinary P4+, serum Ca2+, and VD3, and decreased femoral and lumbar bone mineral density, respectively, in association with renal dysfunction, compared with sham rats. Three weeks of FPS or CTR administration significantly improved serum FGF23 and BMD, as well as urinary Ca2+, P4+, serum Ca2+, P4+, and VD3 levels in CKD-MBD model rats compared with CKD-MBD rats as shown in Figures 1A-E and 2A, B.
Next, they examined the effects of FPS and CTR on femoral lesions in CKD-MBD model rats using histopathological observation and micro-CT analysis. As shown in Figures 2C and D, treatment with FPS or CTR led to a subtle enhancement of osteoporosis-like lesions within the femurs of CKD-MBD model rats, compared to their untreated counterparts. Specifically, the trabecular bone became less thin and disorganized, and the number of lacunae decreased. Accordingly, micro-CT analysis also showed that Tb. Th, Tb.Sp, TV, BV, BV/TV, Tb. N, and mean CT values improved to various degrees in CKD-MBD model rats after FPS or CTR administration compared with the CKD-MBD group.
The study suggests that FPS and CTR could potentially improve calcium and phosphorus metabolic disorders and bone lesions observed in CKD-MBD model rats.
The effects of FPS and CTR on the expression of signaling molecules are important for the FGF23-Klotho signaling pathway (e.g., Klotho, FGF23, FGFR1) in the renal tubulointerstitium and kidneys of CKD-MBD model rats were examined using IHC staining and WB analysis. In CKD-MBD model rats, the induction of renal injury led to a marked decrease in IHC staining for Klotho, FGFR1, and FGF23 within the renal tubulointerstitium. During the same period, the kidneys exhibited markedly lower Klotho protein expression and considerably higher FGFR1 protein expression relative to the sham rat group. Following three weeks of FPS or CTR treatment, Klotho levels increased in the renal tubulointerstitium and kidneys of rats with CKD-MBD compared to untreated CKD-MBD rats, while FGFR1 levels remained unchanged. To further examine whether FPS and CTR affect the expression of key signaling molecules in the FGF23-Klotho signaling pathway in vitro, we exposed cultured NRK-52E cells to TGF-β and rFGF23 for 24 hours and compared the protein expression levels of Klotho and FGFR1 with those of control cells.
The researcher found that rFGF23 decreased Klotho protein expression and increased FGFR1 protein expression in cultured NRK-52E cells exposed to TGF-β for 24 hours compared with control cells. Additionally, the administration of suitable amounts of CTR or FPS for a 24-hour period markedly ameliorated the rFGF23-induced modifications in Klotho and FGFR1 protein expression within cultured NRK-52E cells subjected to TGF-β, in comparison to treatment solely with rFGF23. The ameliorative effect of FPS and CTR on Klotho protein expression was clear. These results indicate that FGF23-Klotho signaling axis in the kidney could be regulated by FPS and CTR in vivo and in vitro.
The ERK1/2-SGK1-NHERF-1-NaPi-2a pathway, downstream of the FGF23-Klotho signaling pathway, plays an important role in phosphate reabsorption in proximal tubular epithelial cells of CKD-MBD model rats. Key signaling molecules in the ERK1/2-SGK1-NHERF-1-NaPi-2a pathway include p-ERK1/2, p-SGK1, NHERF-1, and NaPi-2a. As a result, the effects of FPS and CTR on the protein expression of p-ERK1/2, p-SGK1, NHERF-1, and NaPi-2a in the kidneys of CKD-MBD model rats were assessed through Western blot analysis. Following the induction of renal injury, the expression of p-ERK1/2 and p-SGK1 proteins was markedly reduced, while the expression of NHERF-1 and NaPi-2a proteins was significantly elevated in the kidneys of CKD-MBD model rats when contrasted with sham rats. After 3 weeks of FPS or CTR administration, changes in the protein expression levels of p-ERK1/2, p-SGK1, NHERF-1, and NaPi-2a in the kidneys of CKD-MBD model rats were significantly improved compared with those of CKD-MBD rats.
Similarly, to confirm in vitro whether FPS and CTR affect the expression of key signaling molecules in the ERK1/2-SGK1-NHERF-1-NaPi-2a pathway, cultured NRK-52E cells were exposed to TGF-β and rFGF23 for 24 hours and compared with control cells. rFGF23 reduced the protein expression levels of p-ERK and p-SGK1 and increased the protein expression level of NaPi-2a in cultured NRK-52E cells exposed to TGF-β compared with control cells. Furthermore, treatment with appropriate doses of CTR or FPS for 24 hours significantly ameliorated the rFGF23-induced changes in the protein expression levels of p-ERK, p-SGK1, and NaPi-2a in cultured NRK-52E cells exposed to TGF-β, compared with rFGF23 treatment. These findings suggest that the ERK1/2-SGK1-NHERF-1-NaPi-2a pathway within the kidney could be subject to regulation by FPS and CTR in both in vivo and in vitro settings.
Klotho is a key upstream factor in the ERK1/2-SGK1-NHERF-1-NaPi-2a pathway. In order to determine if restoring Klotho deficiency through FPS helps activate the ERK1/2-SGK1-NHERF-1-NaPi-2a pathway in a laboratory setting, researchers treated NRK-52E cells with TGF-β and rFGF23 for a 24-hour period. The effect of FPS on the protein expression of p-ERK1/2 and p-SGK1, identified as pivotal signaling molecules in the activation of the ERK1/2-SGK1-NHERF-1-NaPi-2a pathway, was subsequently analyzed in comparison to cells transfected with a control. Short hairpin RNA (shRNA) plasmids were also designed by them, and they proved effective in decreasing Klotho protein expression when transfected into NRK-52E cells.
Experiments were then performed utilizing both control and Klotho-specific shRNA plasmids in NRK-52E cells. The results showed that the basal levels of p-ERK1/2 and p-SGK1 were reduced in cells transfected with the shRNA-Klotho plasmid compared with cells transfected with the control plasmid, indicating that Klotho can regulate their basal expression. In cells transfected with the control plasmid, FPS considerably lowered the TGFβ-induced overexpression of p-ERK1/2 and p-SGK1. However, these positive results were almost completely undone when Klotho was silenced using shRNA. The findings suggest that FPS might precisely activate the ERK1/2-SGK1-NHERF-1-NaPi-2a pathway by counteracting Klotho loss in laboratory settings.
The research convincingly showed that FPS, a natural inhibitor of kidney function that resembles CTR, alleviates calcium and phosphorus metabolic issues and bone problems linked to kidney damage in CKD-MBD model rats. The researchers also showed that the positive impacts of FPS on phosphorus absorption, both in living organisms and in laboratory settings, are strongly linked to the control of the FGF23-Klotho signaling pathway and the ERK1/2-SGK1-NHERF-1-NaPi-2a pathway within the kidney. The study’s findings, supported by pharmacological evidence, demonstrate FPS’s direct impact on CKD-MBD treatment.

Source: Front Pharmacol. 2021 Jan 28; 11:586725. doi: 10.3389/fphar.2020.586725
