Undaria pinnatifida fucoidan (UPF), a bioactive sulfated polysaccharide, is widely known to have anti-inflammatory, antioxidant, antitumor, anticoagulant, antiviral, and immunomodulatory properties. The exact mechanism by which UPF regulates inflammation and neuronal health is unclear.
In this blog, I would like to inform you of the study,” Neuroprotective and Anti-Inflammatory Activity of Undaria pinnatifida Fucoidan In Vivo—A Proteomic Investigation” by Cheng Yang et al, which aimed to investigate the effects of UPF supplementation on pro-inflammatory cytokines in skeletal muscle, small intestine, and hypothalamus, as well as plasma cytokine concentrations.
First, to evaluate the effects of UPF on neuronal protein expression in mice, we performed brain proteome analysis in the nucleus accumbens (NAc). A total of 64 C57BL/6J mice were fed a standard or high-fat diet (HFD) with or without UPF (400 mg/kg/day) for 10 weeks. HFD consumption increased the mRNA expression of TNF-α, IL-1β, and IL-6 in muscle compared with mice fed a standard diet. However, UPF supplementation significantly reduced the mRNA expression of TNF-α, IL-1β, and IL-6 in HFD-fed mice. UPF supplementation did not significantly affect the mRNA expression of these cytokines in mice fed a standard diet.
Overall, HFD increased the intestinal mRNA expression of TNF-α, IL-1β, IL-6, nuclear factor-κB, Tjp1, and G protein-coupled receptors 41 and 43 (GPR41 and GPR43) compared with standard chow. However, UPF supplementation significantly decreased the mRNA expression of TNF-α, IL-1β, IL-6, NF-κB, Tjp1, GPR41, and GPR43 in mice fed an HFD. No significant effect of UPF supplementation on these genes was observed in mice fed a standard chow.
Compared with mice that fed a standard chow diet, HFD feeding significantly increased the mRNA expression of TNF-α and IL-1β in the hypothalamus. In HFD-fed mice, UPF supplementation significantly reduced the mRNA expression of TNF-α and IL-1β. Furthermore, UPF administration significantly reduced the expression levels of IL-6 and IFN-γ in the hypothalamus. In contrast, UPF supplementation had no statistically significant effect on the mRNA expression of TNF-α, IL-1β, IL-6, or IFN-γ in mice fed a chow diet.
Also, HFD feeding increased plasma IL-1α concentrations compared to the standard chow group. UPF supplementation significantly reduced IL-1α concentrations by 61.7% in HFD-fed mice, but no statistically significant effect was observed in the chow group. Furthermore, UPF supplementation significantly reduced plasma IL-6 concentrations by 73.9% in HFD-fed mice, but no effect was observed in the chow group. Although UPF supplementation led to a reduction in TNF-α levels, this effect was not statistically significant. No statistically significant changes were observed in mice consuming a chow diet.
A total of 5,423 proteins were identified in the NAc of mice fed either an HFD or standard chow diet. In the CHOW group, 23 proteins showed significant differences in expression between the UPF-treated and control groups. In the HFD group, UPF significantly regulated the expression of 36 proteins compared to the control group. A volcano plot highlights these differences, showing proteins with a log2 fold change (FC) greater than ±0.57 and an FDR-adjusted p-value less than 0.05.
Of the 23 DEPs in the CHOW group, three proteins were up-regulated, and 20 proteins were down-regulated in the NAc of UPF-treated mice. In the HFD group, four proteins were up-regulate,d and 32 proteins were down-regulated in the NAc of UPF-treated mice.
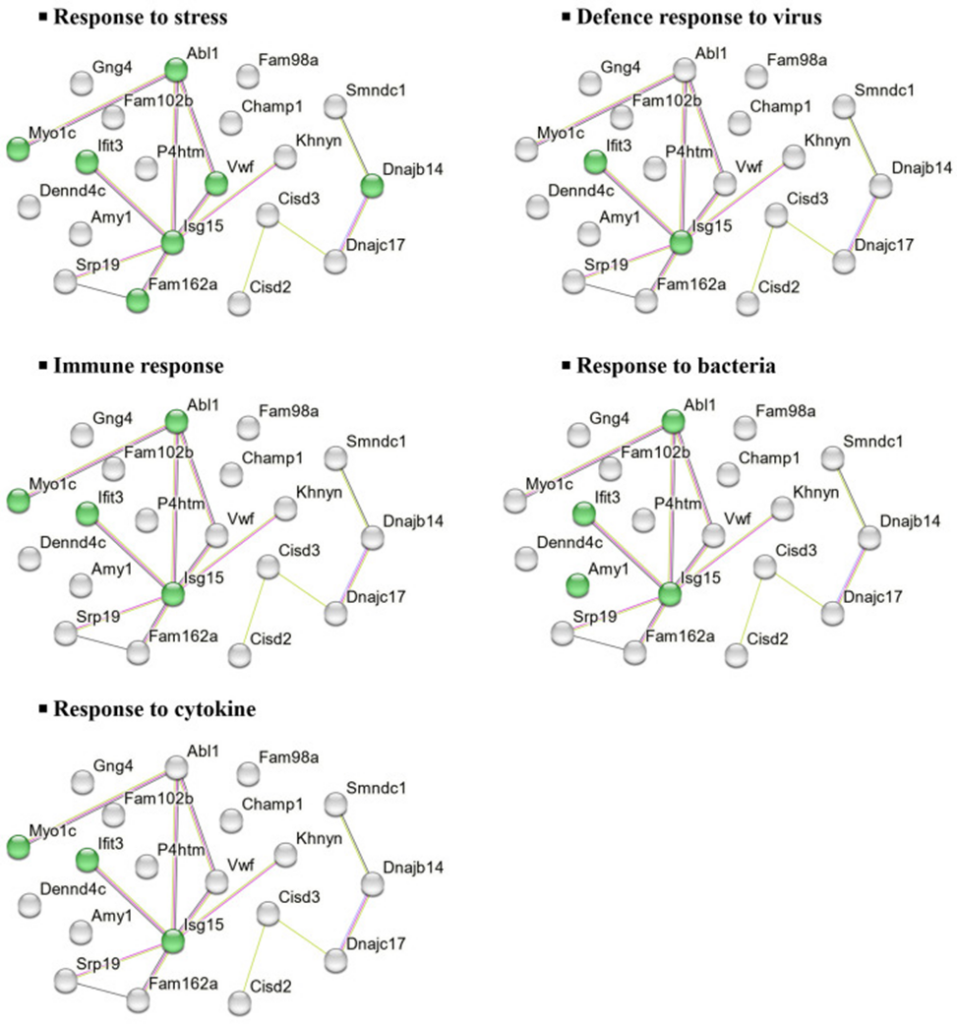
To further investigate the molecular functions and biological significance of these DEPs, we performed in silico analysis using STRING, a protein-protein interaction (PPI) network tool. This analysis revealed major biological processes associated with 20 DEPs downregulated in the CHOW group, including stress response, viral defense, cytokine response, immune response, and bacterial defense (Figure 1).
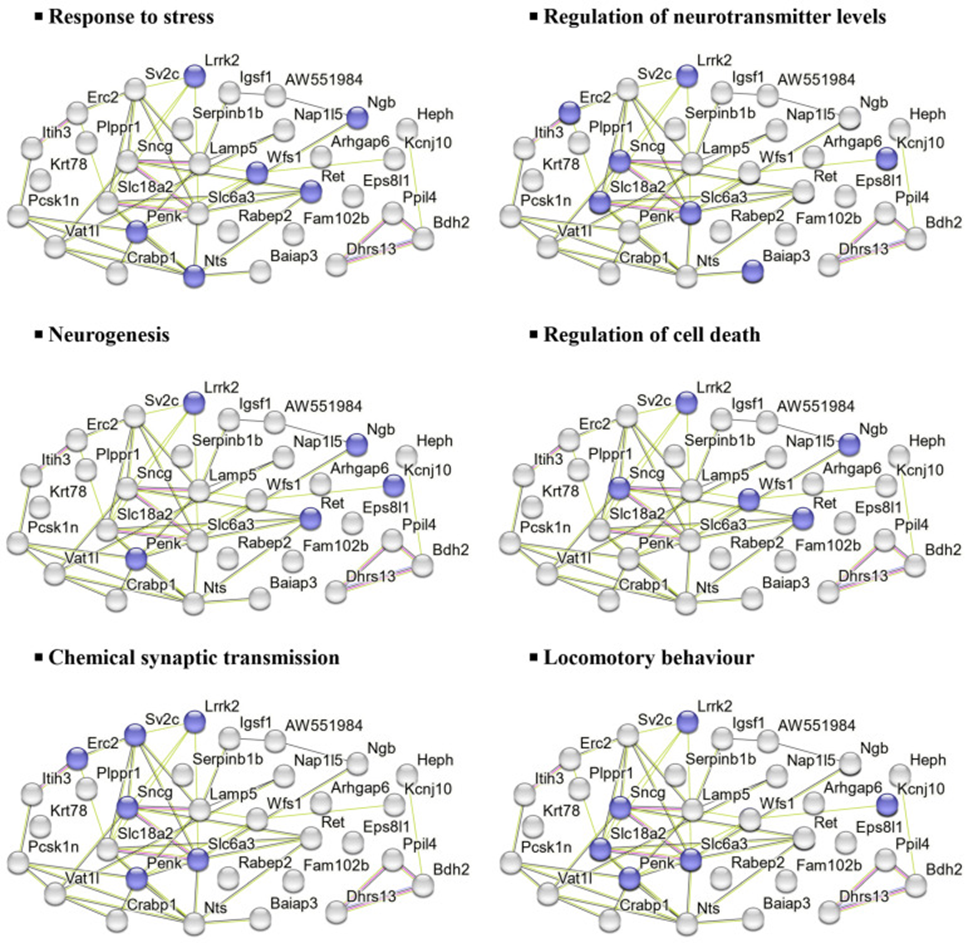
Similarly, the 32 DEPs downregulated in the HFD group were associated with processes such as stress response, neurotransmitter regulation, neurogenesis, cell death regulation, and synaptic transmission, as shown in Figure 2.
Furthermore, of the three DEPs elevated in the NAc of mice fed a standard diet, Cenpv and Nup210 were associated with the biogenesis and organization of cellular components. At the same time, no PPIs were detected in Cops2. On the other hand, of the four DEPs elevated in mice fed an HFD, Ppm1j and Mlip were involved in cellular metabolic processes. However, no PPIs were detected in Fhl2 and Yjefn3.
They also used DAVID to explore the biological processes and functions of elevated and decreased DEPs. In the CHOW group, elevated DEPs were exclusively found in the nuclear envelope and its cellular components. Meanwhile, decreased DEPs were associated with type II interferon production, stress response, bacterial defense, and iron-sulfur cluster binding. In the HFD group, elevated DEPs were primarily associated with biological processes, including RNA polymerase II transcription regulation, DNA template transcription, and RNA biogenesis, as well as molecular functions such as transcriptional corepressor and coregulator activity. No changes were observed in cellular components. On the other hand, decreased DEPs were mainly related to signal transduction, neurotransmitter transport regulation, oxidative stress, and synapse-related functions.
The findings demonstrate the ability to attenuate inflammation and modulate stress-related pathways in HFD-fed mice, while exerting distinct immune-related effects in chow-fed mice. The observed changes in cytokine expression and proteomic profile suggest that UPF may have neuroprotective support for inflammation-related diseases and brain health, especially under metabolic stress.
Source: Mar Drugs. 2025 Apr 27;23(5):189. doi: 10.3390/md23050189
