Chronic kidney disease (CKD), a condition characterized by persistent abnormalities in the structure and function of the kidney, can be attributed to a range of different factors. Over the past decade, CKD has become a significant cause of morbidity and mortality worldwide, with a cumulative mortality rate reaching 20.6%. Cognitive problems in individuals with CKD have become a subject of heightened interest in recent times.
In contrast, studies into alternate treatments have been performed. Fucoidan, which is derived from Laminaria Japonica, exhibits a variety of biological actions, such as neuroprotection, antitumor effects, anti-inflammatory capabilities, antiviral properties, antioxidant characteristics, anticoagulant functions, lipid-lowering effects, and antithrombotic activities. However, it is uncertain if fucoidan can enhance memory problems caused by adenine-induced CKD.
This blog post will discuss the research paper, “The Emerging Evidence for a Protective Role of Fucoidan from Laminaria japonica in chronic kidney disease-Triggered Cognitive Dysfunction” by Zhihui Ma et al.
The initial evaluation of kidney failure relies heavily on assessing high uric acid and urea levels. The kidneys’ ability to function can be helpfully assessed using creatinine, which is the nitrogen-containing waste generated at the end of protein metabolism. Chronic kidney disease is characterized by a decline in renal function, which is evidenced by increased levels of urea, creatinine, and uric acid; it also leads to significant histological changes like damage to the glomeruli, necrosis of the tubules, and the migration of inflammatory cells into the kidney.
As a result of their investigation, they found that the levels of uric acid, urea, and creatinine were higher than expected. Mice given adenine had notably higher levels of uric acid and urea in their urine compared to the control group, and their serum creatinine levels also showed a tendency to rise. Meanwhile, administration of fucoidan (100 mg kg-1 d-1 and 200 mg kg-1 d-1) and losartan dramatically reduced uric acid, urea, and creatinine levels. In addition, administering a comparatively small dose of fucoidan (10 mg kg-1 d-1) to individuals experiencing adenine-induced chronic kidney disease and memory issues led to a notable improvement in both kidney and cognitive function.
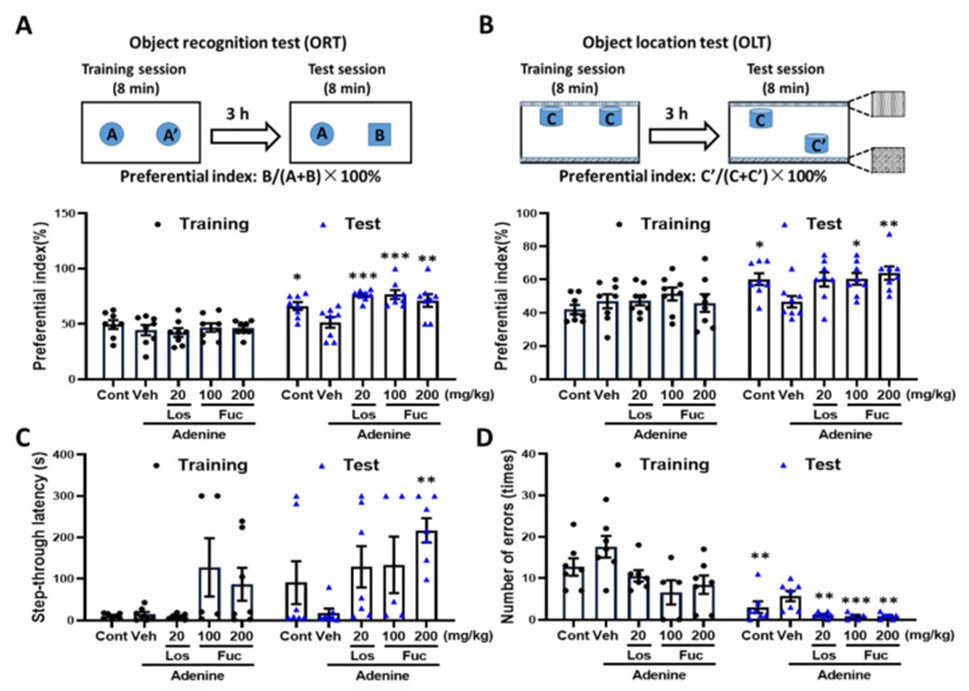
Mice were tested using a novel object recognition memory test to investigate how fucoidan impacts CKD-related recognition memory issues; during this test, they explored identical objects labeled A and A’ for the same amount of time. The administration of fucoidan and losartan during the test session dramatically increased the exploration time for novel object B when compared to the control mice; this trend also manifested in the control group, which suggests improved recognition memory (See Figure 1A).
The study utilized an object location memory test to determine if fucoidan enhances spatial memory. During the training session, the preference index, as shown in Figure 1B, was observed to be roughly 50% when comparing the different groups. In the test session, object location memory was significantly impaired in CKD mice compared with the control group, but was fully restored after administration of fucoidan and losartan (see Figure 2B). When subjected to a passive avoidance memory test, the control mice exhibited a reduced step-through latency when entering the dark compartment, along with a statistically significant increase in the number of errors committed within the dark compartment, relative to the normal control group, as illustrated in Figure 1C and D. Administration of fucoidan and losartan prolonged step-through latency and dramatically reduced the number of errors (see Figure. 1C, D).
These findings, taken as a whole, imply that fucoidan taken by mouth can reverse cognitive problems in mice with chronic kidney disease caused by adenine. To understand the key elements contributing to memory problems caused by chronic kidney disease (CKD), we conducted an RNA-Seq analysis using mice with age-related CKD, which simulates the CKD seen in humans. ELISA analysis of uric acid and urea revealed significantly increased expression in aged mice compared with young mice. Compared to normal young mice, 307 genes were upregulated and 189 genes were downregulated in the brain. Meanwhile, 835 genes were upregulated and 403 genes were downregulated in the kidney.
GO annotation and enrichment analysis were employed to examine signaling pathways within the brain-renal axis that play a role in memory problems associated with CKD. The differentially expressed genes between aged CKD mice and normal young mice were used for GO enrichment analysis using the DAVID database. Analysis of GO annotations showed that molecular functions in the kidney and brain, such as antioxidant activity, binding, cargo receptor activity, and catalytic activity, were regulated. Based on GO enrichment analysis, 30 key biological processes were significantly implicated, including several inflammation-related signaling pathways, like immune system response, inflammatory response, B cell-mediated immunity, innate immune response, and cytokine production regulation, which were extensively modulated in the brains and kidneys of mice with age-related kidney failure.
The interplay between the kidneys and the brain, particularly in the context of both sudden and long-term kidney damage, is significantly influenced by damage caused by cytokines and the presence of oxidative stress. Malondialdehyde (MDA), an end product of lipid oxidation, was dramatically increased in the brain hippocampus and kidney of CKD mice compared with control mice. Following fucoidan and losartan treatment, MDA expression levels were significantly restored. Activation of key antioxidant enzymes, GSH-Px and SOD, is an important therapeutic strategy for treating diseases characterized by elevated oxidative stress. The activity of GSH-Px and SOD in the hippocampus and kidney was noticeably lower in CKD mice, and this reduction was less pronounced in the hippocampus and kidney of CKD mice.
The study aimed to determine if the GSK3β-Nrf2-HO-1 signaling pathway played a role in the antioxidant effects of fucoidan. GSK3β mRNA expression was significantly increased in both the hippocampus and kidney of the vehicle group compared with the control group, and this was improved after fucoidan and losartan treatment. Nuclear factor E2-related factor 2 (Nrf2), a redox-sensitive transcription factor, is thought to be a downstream target of GSK-3β. Compared to the control group, mRNA expression of Nrf2, HO-1, and NQO1 in the hippocampus and kidney was significantly decreased in the control group, but was restored by fucoidan administration.
In both sudden and long-lasting kidney damage, the way the kidneys and brain communicate is greatly affected by cytokine-caused injury and oxidative stress. Malondialdehyde (MDA), an end product of lipid oxidation, was dramatically increased in the brain hippocampus and kidney of CKD mice compared with control mice. Following fucoidan and losartan treatment, MDA expression levels were significantly restored. When treating diseases that are characterized by a high degree of oxidative stress, a key therapeutic strategy involves the activation of essential antioxidant enzymes, including GSH-Px and SOD. In the hippocampus and kidney of mice with chronic kidney disease, the activity of GSH-Px and SOD showed a significant decline, which was then attenuated in the hippocampus and kidney of CKD mice, respectively.
They tested whether the GSK3β-Nrf2-HO-1 signaling pathway was involved in the fucoidan-mediated antioxidant effect. GSK3β mRNA expression was significantly increased in both the hippocampus and kidney of the vehicle group compared with the control group, and this was improved after fucoidan and losartan treatment. Nuclear factor E2-related factor 2 (Nrf2), a redox-sensitive transcription factor, is thought to be a downstream target of GSK-3β. In comparison to the control group, the mRNA expression of Nrf2, HO-1, and NQO1 within the hippocampus and kidney exhibited a significant reduction, although this effect was subsequently reversed following the administration of fucoidan.
The study aimed to determine if fucoidan has any influence on the body’s inflammatory cytokine release process. They found that fucoidan significantly inhibited the mRNA expression of TNF-α and IL-1β and promoted the mRNA expression of the anti-inflammatory cytokine IL-4 in the hippocampus and kidneys of adenine-induced CKD mice. Fucoidan downregulated the mRNA expression of M1 microglia/macrophage-associated iNOS in the hippocampus, while upregulating the mRNA expression of M2 microglia/macrophage-associated TGFβ, Arg1, and CD206 in the hippocampus and kidney.
According to these results, fucoidan appears to be exerting its anti-inflammatory effects by both preventing the formation of M1 microglia/macrophages and by helping to promote the creation of M2 microglia/macrophages.
They conducted a further analysis of gene expression variations between elderly CKD mice and young, healthy mice to shed light on the characteristics of memory impairment brought on by CKD. In the brain, Alzheimer’s disease- and memory-related genes (Camk2n1, Apod, Serpina3n) and immune response-related genes (Spp1, CD68, GFAP, Mpeg, Ddx3y, Lyz2, CTSZ, Tyrobp, C4b, C1qa) were selected for mRNA qPCR analysis. In the kidney, catalytic and metabolic function-related genes (Acy3, Angptl7), ion transporters (Slc22a12, Slco1a1, Slc7a13), and immune response-related genes (Cxcl13, Chd9, Spp1) were analyzed. A Pearson correlation was used to assess the association between gene expression and novel object recognition memory. The hippocampal Camk2n1 gene showed a significant positive correlation with cognitive behavior, whereas SPP1, Cd68, GFAP, Apod, and Serpina3 showed a negative correlation. It was also found that kidney Acy3 and Slc22a12 correlated positively with cognitive behavior, but Angptl7 and Cxcl13 correlated negatively.
The study suggests that fucoidan treatment improved adenine-induced kidney damage and memory impairment, probably by affecting oxidative signaling (GSK3β-Nrf2-HO-1) and inflammatory pathways connected to microglia/macrophage polarization. The genes Acy3, Slc22a12, Angpt7, and Cxcl13 were pinpointed as signature genes that contribute to the memory issues stemming from CKD. This research helps clarify how cognitive problems arise in those with chronic kidney disease (CKD), suggests new treatment approaches, and offers an experimental foundation for using fucoidan to potentially lessen CKD-related memory issues.
Source: Mar Drugs. 2022 Apr 7;20(4):258. doi: 10.3390/md20040258
