Mitochondrial dysfunction is thought to play a key role in dopamine neuron degeneration in patients with sporadic and familial Parkinson’s disease (PD), so approaches aimed at preserving mitochondrial function may hold great promise in the prevention and treatment of PD. In this blog, it presents the study, “Fucoidan Protects Dopaminergic Neurons by Enhancing the Mitochondrial Function in a Rotenone-induced Rat Model of Parkinson’s Disease” by Li Zhang et al that investee the protective effects of fucoidan, a sulfated polysaccharide derived from kelp (Laminaria japonica), on the dopamine system and mitochondrial function of dopamine neurons in a rotenone-induced PD rat model.
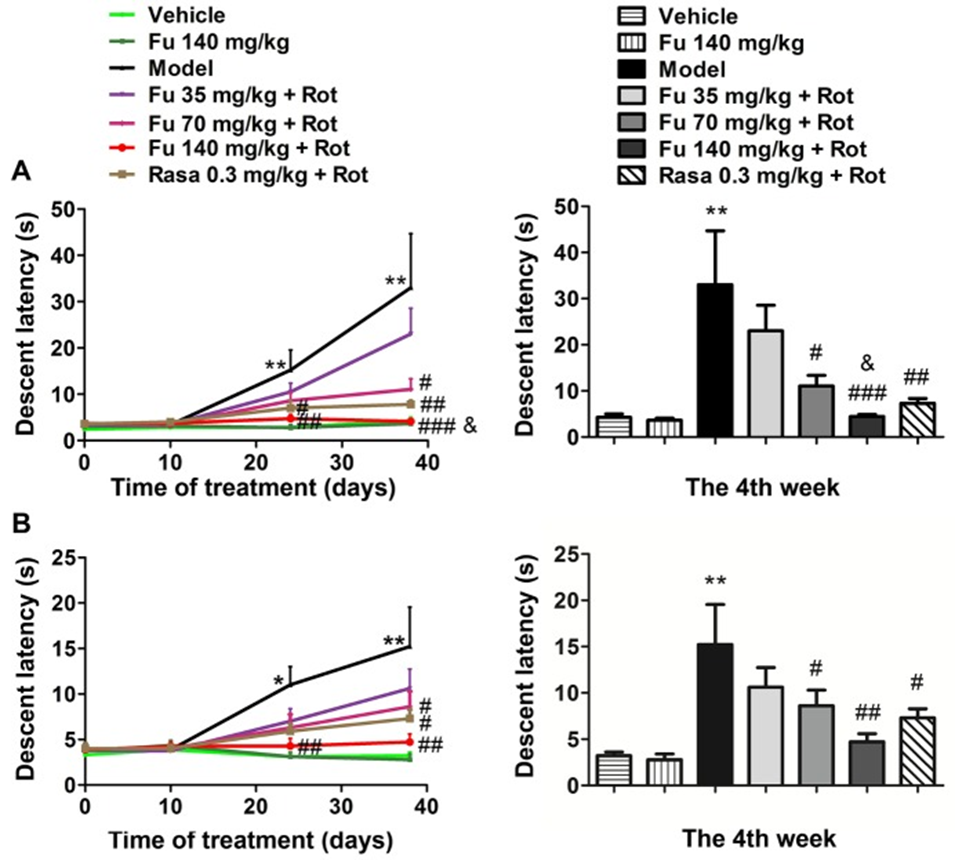
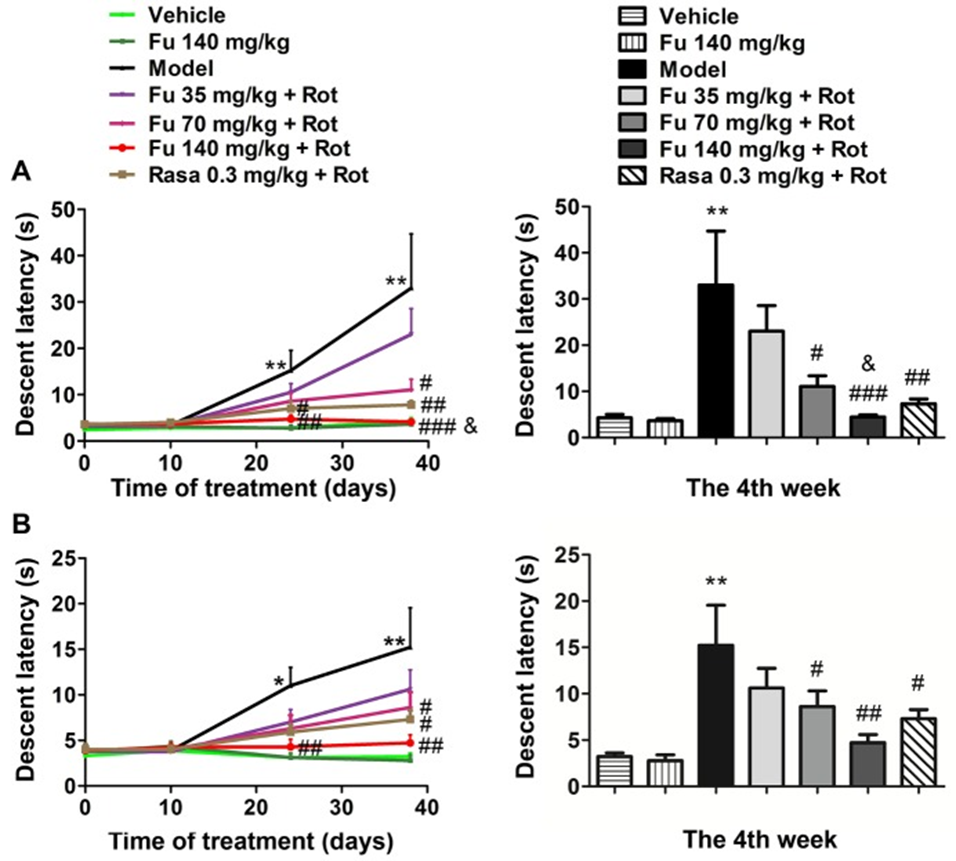
The descending latency in the bar (Fig. 1A) and grid test (Fig. 1B) during the acquisition test at four different time points was measured, and the rats in the model group showed a significant suppression of the prolongation of the descending latency by fucoidan pretreatment compared with the control group (vehicle only). The effect of fucoidan was dose-dependent (35, 70, and 140 mg/kg). At the fourth week, the two higher doses of fucoidan (70 and 140 mg/kg) significantly shortened the descending latency, while the lower dose of fucoidan (35 mg/kg) had no effect. The monoamine oxidase inhibitor rasagiline also improved the cataleptic behavior induced by rotenone (group 7), which was used as a positive control. The administration of 140 mg/kg of fucoidan shortened the descending latency in the bar test more than the rasagiline group. The administration of fucoidan alone did not induce cataleptic behavior in normal rats.
In this study, they measured various locomotor activities, including FP movement, movement time, movement distance, and average speed. As shown in Figure 2, rats treated with rotenone consistently showed significant decreases in all four types of locomotor activity compared with vehicle-treated rats. Pre-administration of fucoidan and rasagiline significantly alleviated the decrease in the four types of locomotor activity. Three doses of fucoidan (35, 70, and 140 mg/kg) significantly restored the decrease in FP movement (Fig. 2A) and FP movement time (Fig. 3B). A high dose of fucoidan (140 mg/kg) restored the decrease in FP movement distance (Fig. 2C) and FP average speed (Fig. 2D), while the two lower doses (35 and 70 mg/kg) did not. Administration of fucoidan alone did not affect locomotor activity in normal rats.
To determine whether fucoidan prevented rotenone-induced loss of nigral DA neurons and striatal DA fibers, immunohistochemistry was performed to monitor changes in TH immunoreactivity in the SNpc and striatum. Rotenone caused a significant loss of TH-positive neurons in the SNpc. Rotenone also caused a significant decrease in TH-positive fibers in the striatum. Three doses of fucoidan pretreatment reduced the loss of TH-positive neurons in the SNpc. At least two higher doses of fucoidan (70 and 140 mg/kg) also reduced the loss of TH-positive fibers in the striatum. Fucoidan alone (group 2) had minimal effects on TH-positive neurons in the SNpc and TH-positive fibers in the striatum.
Changes in the amounts of DA and its metabolites in the striatum were measured by HPLC-ECD. Administration of rotenone alone significantly decreased DA and DOPAC. Compared with vehicle-treated rats, rotenone-treated rats did not change HVA concentrations, but the turnover rate of DA, calculated as the ratio of (DOPAC + HVA) to DA, significantly increased. Notably, three doses of fucoidan inhibited the decrease in DA and DOPAC induced by rotenone. Furthermore, 140 mg/kg fucoidan reversed the increase in the turnover rate of DA in response to rotenone. Fucoidan alone did not change the levels of DA and its metabolites in the striatum.
Electron microscopy of mitochondrial ultrastructure in neuronal cell bodies located in the ventral midbrain showed that mitochondria from rats treated with vehicle or fucoidan alone exhibited normal structure with a dense matrix and regular cristae. In contrast, mitochondria from rats treated with rotenone were swollen and had reduced fragmented cristae. The matrix was almost absent. Mitochondria from rats treated with fucoidan and rotenone were relatively normal, rod-shaped, with dense and regular cristae.
Mitochondrial respiratory function was assessed by analyzing multiple parameters, including basal mitochondrial respiration, ATP production, maximal respiration, and residual oxygen consumption. Fucoidan alone had no significant effect on mitochondrial respiratory function, whereas mitochondrial respiratory function was significantly decreased in rats receiving rotenone alone. Three different doses of fucoidan dose-dependently attenuated the rotenone-induced decrease in basal respiration, ATP production, and maximal respiration. Fucoidan at 140 mg/kg completely restored the levels of these three parameters, and its beneficial effects on ATP production and maximal respiration were superior to rasagiline. No significant differences in residual oxygen consumption were observed between the different groups, suggesting that the effects of fucoidan on respiratory function are not due to non-mitochondrial oxygen consumption.
To elucidate the function of mitochondrial complexes, we tested the activity of mitochondrial complexes I and II in ventral midbrain tissue from permeabilized rats. Basal respiration and complex I activity were decreased in ventral midbrain tissue in rats treated with rotenone compared to vehicle-treated rats. Fucoidan at 140 mg/kg dramatically increased basal respiration. Fucoidan at 70 and 140 mg/kg also increased complex I activity. The effect of 70 mg/kg fucoidan on basal respiration and complex I activity was similar to that of rasagiline. Mitochondrial complex II activity showed a tendency to decrease in rotenone-treated rats, but did not reach a statistically significant level. Fucoidan alone did not change basal respiration or complex I and II activity in normal rats.
The oxidative stress status was evaluated by measuring the contents of three oxidative stress products (MDA, 3-NT, and 8-OHdG) in the rat ventral midbrain. Rotenone significantly increased the concentrations of MDA, 3-NT, and 8-OHdG (Fig. 8C). In the presence of fucoidan, the increase in MDA concentration by rotenone was significantly inhibited. Fucoidan completely inhibited the rotenone-induced increase in 3-NT and 8-OHdG concentrations, and its effect on 3-NT was superior to that of rasagiline. Fucoidan alone had no significant effect on the basal concentrations of the three products in normal rats.
Western blot analysis was performed to examine the expression changes of two proteins related to mitochondrial function, PGC-1α and NRF2.The expression level of PGC-1α in the ventral midbrain was decreased in PD model rats (treated with rotenone alone) compared with vehicle-treated rats. Pretreatment with fucoidan at three different doses significantly restored the level of PGC-1α. Similar results were obtained with NRF2 expression analysis. Rasagiline also restored the decreased expression of PGC-1α and NRF2 induced by rotenone. Treatment with fucoidan alone did not change the basal levels of PGC-1α and NRF2 proteins in the ventral midbrain of normal rats.
These results indicate that fucoidan can protect the dopamine system in PD rats. The neuroprotective effect of fucoidan may be mediated through the maintenance of mitochondrial function involved in the PGC-1α/NRF2 pathway, providing new evidence that fucoidan may be useful in the treatment of PD.
Western blot analysis was performed to examine the expression changes of two proteins related to mitochondrial function, PGC-1α and NRF2. The expression level of PGC-1α in the ventral midbrain was decreased in PD model rats (treated with rotenone alone) compared with vehicle-treated rats. Pretreatment with fucoidan at three different doses significantly restored the level of PGC-1α. Similar results were obtained with NRF2 expression analysis. Rasagiline also restored the decreased expression of PGC-1α and NRF2 induced by rotenone. Treatment with fucoidan alone did not change the basal levels of PGC-1α and NRF2 proteins in the ventral midbrain of normal rats.
These results indicate that fucoidan can protect the dopamine system in PD rats. The neuroprotective effect of fucoidan may be mediated through the maintenance of mitochondrial function involved in the PGC-1α/NRF2 pathway, providing new evidence that fucoidan may be useful in the treatment of PD.
Source:Aging Dis. 2018 Aug 1;9(4):590–604. doi: 10.14336/AD.2017.0831
