Mitochondria are the most commonly damaged organelles in dopaminergic neurons in Parkinson’s disease (PD) patients. Despite the importance of protecting neuronal mitochondria in PD patients, the detailed mechanisms of mitochondrial dysfunction in the development and pathophysiological progression of PD remain to be elucidated.
So, in this blog, I would like to inform of the study, “Fucoidan Suppresses Mitochondrial Dysfunction and Cell Death against 1-Methyl-4-Phenylpyridinum-Induced Neuronal Cytotoxicity via Regulation of PGC-1α Expression” by Yong-Seok Han et al that investigated the protective effect of fucoidan against the deleterious effects of 1-methyl-4-phenyl-pyridinium (MPP+), a neurotoxin used as a model of PD, on mitochondria in SH-SY5Y neuronal cells.
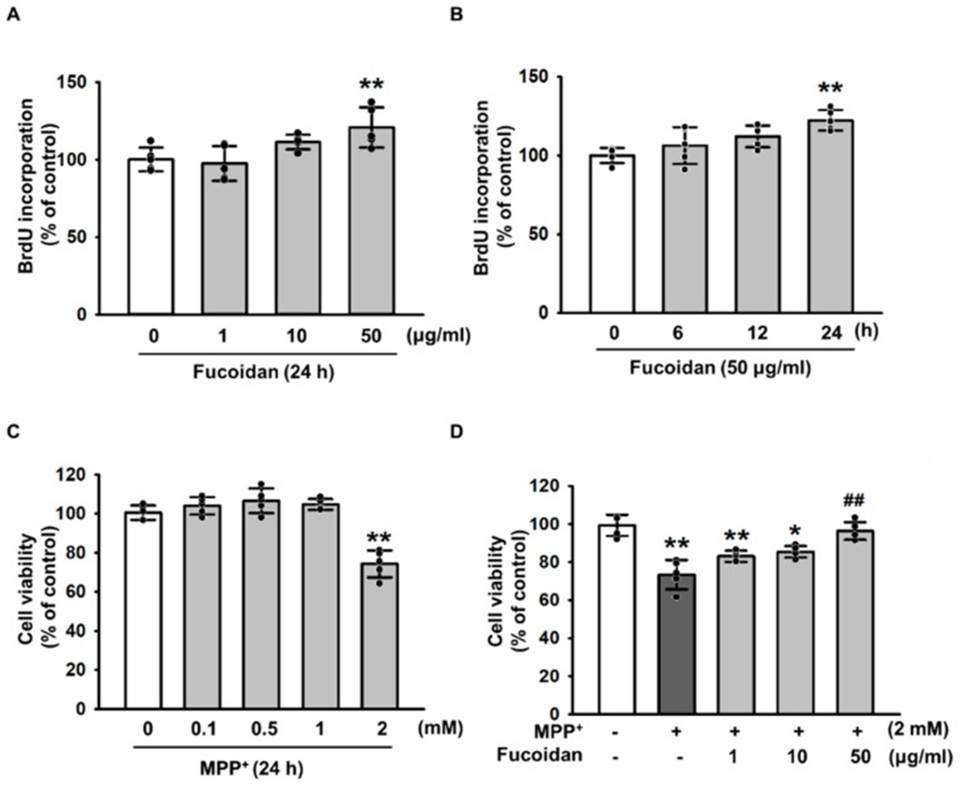
First, to investigate the protective effect of fucoidan against MPP+-induced oxidative stress-induced cytotoxicity, they treated SH-SY5Y cells with various concentrations of fucoidan. As a result, fucoidan stimulated cell proliferation at 50 μg/mL after 24 h (Fig. 1A, B). Furthermore, we treated SH-SY5Y with MPP+ for the indicated dose periods (0, 0.1, 0.5, 1, and 2 mM), and the MPP+-induced maximum decrease in SH-SY5Y viability was observed at 2 mM (Fig. 1C). However, the decrease in viability due to MPP+-induced apoptosis was prevented by pretreatment with fucoidan (Fig. 1D). These results indicate that fucoidan protects SH-SY5Y cells from MPP+-induced apoptosis. These findings suggest that fucoidan increases SH-SY5Y cell proliferation and cell viability in the MPP+-induced PD model.
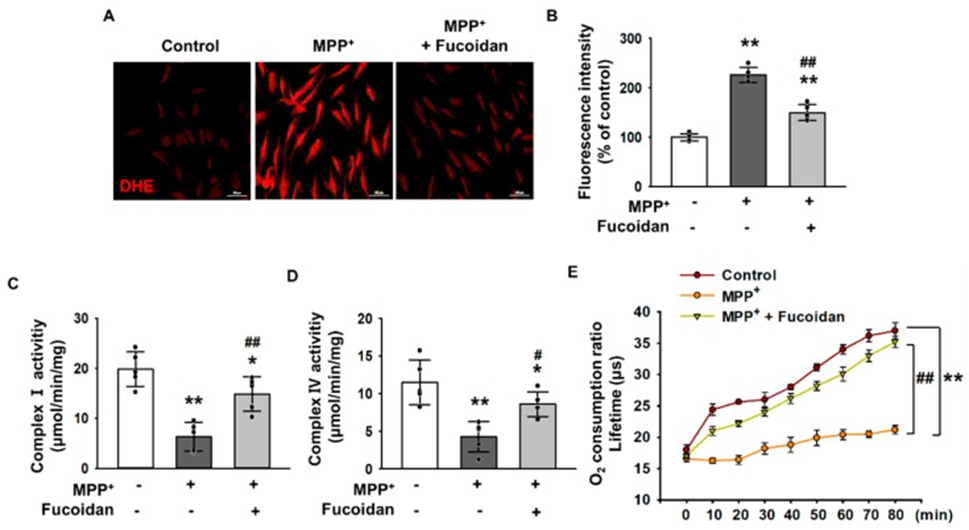
Next, to investigate the effect of fucoidan on oxidative stress in response to MPP+ treatment, MPP+-treated cells were incubated with dihydroethidium (DHE) for ROS detection, and the changes in oxidative stress levels were measured using fluorescence microscopy images. SH-SY5Y cells exposed to 2 mM MPP+ for 24 h had significantly higher oxidative stress levels compared to untreated cells, indicating that MPP+ enhances oxidative stress (Fig. 2A, B). Fluorescence microscopy images of DHE revealed that the elevated oxidative stress levels were significantly alleviated by fucoidan treatment (Fig. 2A, B). Furthermore, to investigate whether MPP+ reduces mitochondrial membrane potential through oxidative stress, the activities of complexes I and IV (Fig. 2C, D) and mitochondrial O2 consumption rate (Fig. 2E) were measured. The results show that MPP+ reduced the activities of complexes I and IV and the mitochondrial O2 consumption rate. In contrast, fucoidan pretreatment protected the activity of complexes I and IV and the mitochondrial O2 consumption rate from MPP+ (Figure 2C–E), suggesting that fucoidan may affect mitochondrial oxidative phosphorylation under MPP+-induced stress conditions. These results indicate that fucoidan protects SH-SY5Y cells from MPP+-induced oxidative stress and mitochondrial dysfunction.
To investigate the key mediators of fucoidan-protected mitochondrial function, they analyzed the expression of 5′ adenosine monophosphate-activated protein kinase (AMPK) and PGC-1α using Western blot analysis in a dose-dependent manner (0-50 μg/mL). Western blot analysis showed that AMPK phosphorylation was increased in SH-SY5Y cells treated with fucoidan (50 μg/mL). PGC-1α expression was also found to be increased at the same concentration. Furthermore, PGC-1α increased its influx into mitochondria. They also confirmed that fucoidan activates PGC-1α by regulating AMPK phosphorylation using the AMPK inhibitor compound C. As a result, when AMPK phosphorylation was inhibited, fucoidan did not increase the expression of PGC-1α in SH-SY5Y cells. These results indicate that fucoidan controls the expression of PGC-1α, a key factor in mitochondrial biogenesis, through the phosphorylation of AMPK.
They also clarified whether the decrease in mitochondrial membrane potential was due to the decrease in PGC-1α expression, the activity of complexes I and IV, and the mitochondrial oxygen consumption rate were measured. The results showed that when PGC-1α expression was suppressed, fucoidan did not increase the activity of complexes I and IV, and the mitochondrial oxygen consumption rate was also suppressed by the suppression of PGC-1α expression. These results indicate that the effect of fucoidan is to increase PGC-1α expression and reduce dysfunctional mitochondria.
They investigated the effect of fucoidan on apoptosis induction by MPP+, and the expression levels of B-cell lymphoma 2 (BCL2), BCL-2-like protein 4 (Bax), cleaved poly(ADP-ribose) polymerase 1 (PARP-1), and cleaved caspase 3, which are associated with cell survival or apoptosis, were analyzed by Western blot assay. When apoptosis was induced in SH-SY5Y cells by oxidative stress caused by MPP+, fucoidan increased the expression of BCL2, whereas the expression levels of Bax, cleaved PARP-1, and cleaved caspase 3 were significantly decreased. Furthermore, apoptosis was evaluated using propidium iodide (PI)/annexin V staining. As a result, fucoidan reduced apoptosis by modulating the expression of PGC-1α. These results indicate that the effect of fucoidan inhibits apoptosis by increasing the expression of PGC-1α.
In conclusion, the study revealed that crude fucoidan isolated from Fucus vesiculosus protects against mitochondrial dysfunction and apoptosis in a PD cell model exhibiting MPP+-induced mitochondrial dysfunction, high oxidative stress, and reduced viability by regulating the AMPK-PGC-1α pathway, suggesting that fucoidan could be used to enhance the efficacy of therapeutic strategies against neurotoxic disorders in PD.
Source: Mar Drugs. 2019 Sep 2;17(9):518. doi: 10.3390/md17090518
